 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm '5 Gauss Limit' found in 1 term [ ] and 2 definitions [ ] and 2 definitions [ ], (+ 4 Boolean[ ], (+ 4 Boolean[ ] results ] results
| 1 - 5 (of 7) nextResult Pages :  [1] [1]  [2] [2] |  | | |  |  |  |
| 5 Gauss Limit |  |
| |
|
The national regulatory boards decided to limit the threshold for access to MRI areas to 5 gauss.
It is of special interest for the observer of bureaucratic procedures that the 5 gauss safety limit is ten times higher than the average earth magnetic field, but lower than the magnetic field in electric trains such as subways (up to 7 gauss).
For example, the fields measured on the surface of the receiver of a telephone are 35 gauss and of an audio headset 100 gauss. | |  | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | | | | • Share the entry '5 Gauss Limit':    | | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|
A pacemaker is a device for internal or external battery-operated cardiac pacing to overcome cardiac arrhythmias or heart block. All implanted electronic devices are susceptible to the electromagnetic fields used in magnetic resonance imaging. Therefore, the main magnetic field, the gradient field, and the radio frequency (RF) field are potential hazards for cardiac pacemaker patients.
The pacemaker's susceptibility to static field and its critical role in life support have warranted special consideration. The static magnetic field applies force to magnetic materials. This force and torque effects rise linearly with the field strength of the MRI machines. Both, RF fields and pulsed gradients can induce voltages in circuits or on the pacing lead, which will heat up the tissue around e.g. the lead tip, with a potential risk of thermal injury.
Regulations for pacemakers provide that they have to switch to the magnet mode in static magnetic fields above 1.0 mT. In MR imaging, the gradient and RF fields may mimic signals from the heart with inhibition or fast pacing of the heart. In the magnet mode, most of the current pacemakers will pace with a fix pulse rate because they do not accept the heartsignals. However, the state of an implanted pacemaker will be unpredictable inside a strong magnetic field. Transcutaneous controller adjustment of pacing rate is a feature of many units. Some achieve this control using switches activated by the external application of a magnet to open/close the switch. Others use rotation of an external magnet to turn internal controls. The fringe field around the MRI magnet can activate such switches or controls. Such activations are a safety risk.
Areas with fields higher than 0.5 mT ( 5 Gauss Limit) commonly have restricted access and/or are posted as a safety risk to persons with pacemakers.

A Cardiac pacemaker is because the risks, under normal circumstances an absolute contraindication for MRI procedures.
Nevertheless, with special precaution the risks can be lowered. Reprogramming the pacemaker to an asynchronous mode with fix pacing rate or turning off will reduce the risk of fast pacing or inhibition. Reducing the SAR value reduces the potential MRI risks of heating. For MRI scans of the head and the lower extremities, tissue heating also seems to be a smaller problem. If a transmit receive coil is used to scan the head or the feet, the cardiac pacemaker is outside the sending coil and possible heating is very limited. | |  | |
• View the DATABASE results for 'Cardiac Pacemaker' (6).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|

It is important to remember when working around a superconducting magnet that the magnetic field is always on. Under usual working conditions the field is never turned off. Attention must be paid to keep all ferromagnetic items at an adequate distance from the magnet. Ferromagnetic objects which came accidentally under the influence of these strong magnets can injure or kill individuals in or nearby the magnet, or can seriously damage every hardware, the magnet itself, the cooling system, etc..
See MRI resources Accidents.
The doors leading to a magnet room should be closed at all times except when entering or exiting the room. Every person working in or entering the magnet room or adjacent rooms with a magnetic field has to be instructed about the dangers. This should include the patient, intensive-care staff, and maintenance-, service- and cleaning personnel, etc..
The 5 Gauss limit defines the 'safe' level of static magnetic field exposure. The value of the absorbed dose is fixed by the authorities to avoid heating of the patient's tissue and is defined by the specific absorption rate.
Leads or wires that are used in the magnet bore during imaging procedures, should not form large-radius wire loops. Leg-to-leg and leg-to-arm skin contact should be prevented in order to avoid the risk of burning due to the generation of high current loops if the legs or arms are allowed to touch. The patient's skin should not be in contact with the inner bore of the magnet.
The outflow from cryogens like liquid helium is improbable during normal operation and not a real danger for patients.
The safety of MRI contrast agents is tested in drug trials and they have a high compatibility with very few side effects. The variations of the side effects and possible contraindications are similar to X-ray contrast medium, but very rare. In general, an adverse reaction increases with the quantity of the MRI contrast medium and also with the osmolarity of the compound.
See also 5 Gauss Fringe Field, 5 Gauss Line, Cardiac Risks, Cardiac Stent, dB/dt, Legal Requirements, Low Field MRI, Magnetohydrodynamic Effect, MR Compatibility, MR Guided Interventions, Claustrophobia, MRI Risks and Shielding. | | | | | | | | |
• View the DATABASE results for 'MRI Safety' (42).
| | |
• View the NEWS results for 'MRI Safety' (13).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
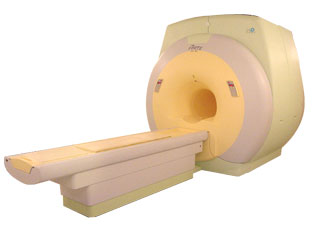
From ISOL Technology
'Ultra high field MR system, it's right close to you.
FORTE 3.0T is the new standard for the future ultra high field MR system.
If you are pushing the limits of your existing clinical MR scanner, the FORTE will surely take you to the next level of diagnostic imaging.
FORTE is the core leader of the medical technology in the 21st century. Proving effects of fMRI that cannot be measured with MRI less than 2.0T.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
128 x 128, 256 x 256, 512 x 512, 1024 x 1024
| |  | |
• View the DATABASE results for 'FORTE 3.0T™' (2).
| | | | |
|  | |  |  |  |
| |
|
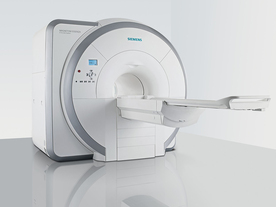
From Siemens Medical Systems;
Received FDA clearance in 2007.
The MAGNETOM Essenza is designed to combine high system performance with simple installation and power requirements to provide optimal operating costs for limited budgets. The standard system has up to 2 5 integrated coil elements and 8 independent radio frequency channels. Tim allows the combination of up to 4 different coils that reduce patient and coil repositioning.
The 1. 5 Tesla system is designated for a complete range of clinical applications, including neurology, orthopedics, body imaging, angiography, cardiology, breast imaging, oncology and pediatric MRI.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, TMJ optional.
CHANNELS (min. / max. configuration)
8, 16
MAGNET WEIGHT (gantry included)
4350 kg in operation
DIMENSION H*W*D (gantry included)
145 x 226 x 216 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
30 mT/m, 300 msec to 10 mT/m
Passive, active; first order standard
second order optional
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 45 kVA
| |  | | | |
|  | |  |  |
|  | 1 - 5 (of 7) nextResult Pages :  [1] [1]  [2] [2] |
| |
|
| |
 | Look
Ups |
| |