 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Arc' found in 6 terms [ ] and 60 definitions [ ] and 60 definitions [ ] ]
| previous 56 - 60 (of 66) nextResult Pages :  [1 2] [1 2]  [3 4 5 6 7 8 9 10 11 12 13 14] [3 4 5 6 7 8 9 10 11 12 13 14] |  | |  | Searchterm 'Arc' was also found in the following services: | | | | |
|  |  |
| |
|
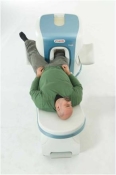
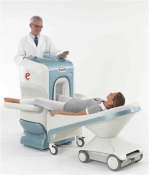
O-scan is manufactured and distributed by Esaote SpA
O-scan is a compact, dedicated extremity MRI system designed for easy installation and high throughput. The complete system fits in a 9' x 10' room, doesn't need for RF or magnetic shielding and it plugs in the wall. The 0.31T permanent magnet along with dual phased array RF coils, and advanced imaging protocols provide outstanding image quality and fast 25 minute complete examinations.
Esaote North America is the exclusive distributor of the O-scan system in the USA.
Device Information and Specification CLINICAL APPLICATION Dedicated Extremity
PULSE SEQUENCES
SE, HSE, HFE, GE, 2dGE, ME, IR, STIR, Stir T2, GESTIR, TSE, TME, FSE STIR, FSE ( T1, T2), X-Bone, Turbo 3DT1, 3D SH ARC, 3D SST1, 3D SST2 2D: 2mm - 10 mm, 3D: 0.6 - 10 mm POWER REQUIREMENTS 100/110/200/220/230/240 | |  | | | |
|  |  | Searchterm 'Arc' was also found in the following services: | | | | |
|  |  |
| |
|
MRI can be indicated for use in pregnant women if other forms of diagnostic imaging are inadequate or require exposure to ionizing radiation such as X-ray or CT.
As a safety precaution, MR scanning should be avoided in the first three months of pregnancy.
Similar considerations hold for pregnant staff of a magnetic resonance department. An epidemiological study (by Kanal, et al.) concluded that data collected from MRI technologists were negative with respect to any statistically significant elevations in the rates of spontaneous abortion, infertility and premature delivery.
However, also for psychological reasons, it might be a wise precaution that pregnant staff members do not remain in the scan room during actual scanning.
There have been several reports (results could not be reproduced) that static magnetic fields may provoke genetic mutations, changes in growth rate and leukocyte count and other effects.
No reports have been published that persons exposed to magnetic fields, including staff at MR departments, have a higher incidence of genetic damage to their children than found in the average population.
This rese arch needs further investigation and for this purpose pregnancy should be considered a relative contraindication for MR spectroscopy and MRI procedures.
Taking into account that clinical MR imaging devices operate at field strengths of between 0.2 and 2.0 T, higher field strengths need more investigation.

| | | |  | |
• View the DATABASE results for 'Pregnancy' (5).
| | |
• View the NEWS results for 'Pregnancy' (1).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
Primovist™ (U.S brand name Eovist®) is a highly specific MRI contrast agent for the imaging, detection and characterization of liver conditions, including liver tumors, cysts, as well as other malignant and benign lesions. It is a water-soluble ethoxybenzyl derivative of Gd-DTPA. This compound is taken up by the hepatocytes (approximately 30% of the dose goes to the hepatocytes) and is equally excreted renal and biliary in humans.
Primovist™ brightens the signal of T1 weighted MR images immediately after contrast administration.
Dynamic scanning and imaging of the accumulation phase (best after 20 min.) can also be performed after bolus injection of Primovistâ„¢. The hepatocytes uptake will increase the signal intensity of normal liver parenchyma. This results in improved lesion-to-liver contrast because malignant tumors (metastases, the majority of hepatocellular c arcinomas) do not contain either hepatocytes or their functioning is hampered.
WARNING:
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate less than 30 mL/min/1.73m 2), or acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period. Drug Information and Specification T1, Predominantly positive enhancement PHARMACOKINETIC 50% hepatobiliary, 50% renal excretion DOSAGE 12,5 - 25 µmol/kg PREPARATION Finished product DEVELOPMENT STAGE for sale DO NOT RELY ON THE INFORMATION PROVIDED HERE, THEY ARE
NOT A SUBSTITUTE FOR THE ACCOMPANYING PACKAGE INSERT! Distribution Information TERRITORY TRADE NAME DEVELOPMENT
STAGE DISTRIBUTOR | |  | |
• View the DATABASE results for 'Primovist™' (7).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Arc' was also found in the following services: | | | | |
|  |  |
| |
|

The range of diagnostics and imaging systems of Siemens Medical Systems covers ultrasound, nuclear medicine, angiography, magnetic resonance, computer tomography and patient monitoring. Siemens is one of the three leading MRI manufacturers, which together account for approximately 80 percent of the MRI machines installed worldwide. Siemens currently offers the Allegra 3T MRI, which is for head scanning only, but the company will also be launching the Trio MRI, a 3T whole body scanner.
Siemens has formed partnerships with more than ten rese arch institutions and private practitioners to define a comprehensive MRI examination and compare MR to currently established cardiovascular modalities, thereby defining optimal diagnosis and treatment.
MRI Scanners:
0.2T to 1.0T:
1.5T:
3.0T to 7.0T:
Hybrid Scanners:
Mobile Solutions:
•
MAGNETOM Espree 1.5T, MAGNETOM Avanto 1.5T and MAGNETOM ESSENZA 1.5T are also offered by Siemens on certified trailers.
Contact Information MAIL
Siemens Medical Solutions
Health Services Corporation
51 Valley Stream Parkway
Malvern, PA 19355
USA | |  | |
• View the DATABASE results for 'Siemens Medical Systems' (14).
| | |
• View the NEWS results for 'Siemens Medical Systems' (3).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Arc' was also found in the following services: | | | | |
|  |  |
| |
|

From GE Healthcare;
GE's Signa Contour/i system uses the innovations like K4 technology and real-time interactive imaging.
This compact magnet with wide-flare gantry obtains high patient comfort with low costs.
Device Information and Specification CLINICAL APPLICATION Whole body Head and body coil standard; all other coils optional; open architecture makes system compatible with a wide selection of coils Standard: SE, IR, 2D/3D GRE and SPGR, Angiography;; 2D/3D TOF, 2D/3D Phase Contrast;; 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, optional: EPI, 2D/3D Fiesta, FGRET, Spiral2D 0.8 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 V STRENGTH SmartSpeed 23 mT/m, HiSpeed Plus 33 mT/m | |  | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |