 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Bo' was also found in the following services: | | | | |
|  |  |
| |
|
Special imaging primarily means advanced MRI techniques used for qualitative and quantitative measurement of biological meta bolism as e.g., spectroscopy, perfusion imaging (PWI, ASL), diffusion weighted imaging ( DWI, DTI, DTT) and brain function ( BOLD, fMRI). This physiological magnetic resonance techniques offer insights into brain structure, function, and meta bolism.
Spectroscopy provides functional information related to identification and quantification of e.g. brain meta bolites.
MR perfusion imaging has applications in stroke, trauma, and brain neoplasm. MRI provides the high spatial and temporal resolution needed to measure blood flow to the brain. arterial spin labeling techniques utilize the intrinsic protons of blood and brain tissue, labeled by special preparation pulses, rather than exogenous tracers injected into the blood.
MR diffusion tensor imaging characterizes the ability of water to spread across the brain in different directions. Diffusion parallel to nerve fibers has been shown to be greater than diffusion in the perpendicular direction. This provides a tool to study in vivo fiber connectivity in brain MRI.
FMRI allows the detection of a functional activation in the brain because cortical activity is intimately related to local meta bolism changes. See also Diffusion Tensor Tractography. | |  | |
• View the NEWS results for 'Special Imaging' (14).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
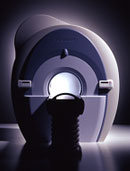
From Toshiba America Medical Systems Inc.;
With its high-field strength, the Vantage™ delivers the clinical capabilities and image quality expected by cardiologists, while simultaneously offering patients a more comfortable and non-invasive option, said Dane Peshe, director, MRI Business Unit, Toshiba America Medical Systems. Vantage™ supports a full complement of cardiovascular imaging studies, ranging from stroke evaluation to peripheral vascular imaging. Additionally, the ultra short bore design offers patients a greater feeling of openness that reduces claustrophobic sensations, while Toshiba's exclusive, patented Pianissimo™ technology reduces scan noise by as much as 90 percent for a more pleasant experience.'
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Ultra short bore SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, EPI, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study 32-1024, phase;; 64-1024, freq. POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Closed-loop water-cooled Liquid helium: approx. less than 0.05 L/hr Passive, active, auto-active | |  | |
• View the DATABASE results for 'Vantage™' (2).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
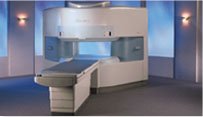
From Hitachi Medical Systems America, Inc.;
the AIRIS made its debut in 1995. Hitachi followed up with the AIRIS II system, which has proven equally successfully. 'All told, Hitachi has installed more than 1,000 MRI systems in the U.S., holding more than 17 percent of the total U.S. MRI installed base, and more than half of the installed base of open MR systems,' says Antonio Garcia, Frost and Sullivan industry research analyst.
Now Altaire employs a blend of innovative Hitachi features called VOSI™ technology, optimizing each sub-system's performance in concert with the
other sub-systems, to give the seamless mix of high-field performance
and the patient comfort, especially for claustrophobic patients, of open MR systems.
Device Information and Specification
CLINICAL APPLICATION
Whole body
DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP)
SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 3.6 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 8 - 250msec IR: 5.2 -7,680msec GE: 1.8 - 2,000 msec FSE: 5.2 - 7,680
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
COOLING SYSTEM TYPE
Water-cooled
3.1 m lateral, 3.6 m vertical
| |  | |
• View the DATABASE results for 'Altaire™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Bo' was also found in the following services: | | | | |
|  |  |
| |
|
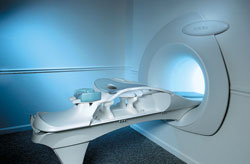
From Aurora Imaging Technology, Inc.;
The Aurora® 1.5T Dedicated Breast MRI System with Bilateral SpiralRODEO™ is the first and only FDA approved MRI device designed specifically for breast imaging. The Aurora System, which is already in clinical use at a growing number of leading breast care centers in the US, Europe, got in December 2006 also the approval from the State Food and Drug Administration of the People's Republic of China (SFDA).
'Some of the proprietary and distinguishing features of the Aurora System include: 1) an ellipsoid magnetic shim that provides coverage of both breasts, the chest wall and bilateral axillary lymph nodes; 2) a precision gradient coil with the high linearity required for high resolution spiral reconstruction;; 3) a patient-handling table that provides patient comfort and procedural utility; 4) a fully integrated Interventional System for MRI guided biopsy and localization; and 5) the user-friendly AuroraCAD™ computer-aided image display system designed to improve the accuracy and efficiency of diagnostic interpretations.'
Device Information and Specification
CONFIGURATION
Short bore compact
TE
From 5 ms for RODEO Plus to over 80 ms, 120 ms for T2 sequences
Around 0.02 sec for a 256x256 image, 12.4 sec for a 512 x 512 x 32 multislice set
20 - 36 cm, max. elliptical 36 x 44 cm
POWER REQUIREMENTS
150A/120V-208Y/3 Phase//60 Hz/5 Wire
| |  | |
• View the DATABASE results for 'Aurora® 1.5T Dedicated Breast MRI System' (2).
| | |
• View the NEWS results for 'Aurora® 1.5T Dedicated Breast MRI System' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
Cytogen Corporation of Princeton, NJ is a product-driven, oncology-focused biopharmaceutical company. Cytogen markets several products through its in- house oncology sales force: ProstaScint® (a monoclonal anti body-based imaging agent used to image the extent and spread of prostate cancer); BrachySeed™ I-125 and BrachySeed™ Pd-103 (two uniquely designed, next generation radioactive seed implants for the treatment of localized prostate cancer), and NMP22® BladderChek™ (a highly accurate and convenient anti body-based point-of-care staging test for bladder cancer detection). Cytogen has also developed Quadramet®, a skeletal targeting therapeutic radiopharmaceutical for the relief of bone pain in prostate and other types of cancer. Cytogen's pipeline comprises product candidates at various stages of clinical development, including fully human monoclonal antibodies and cancer vaccines based on PSMA (prostate specific membrane antigen) technology, which was exclusively licensed from Memorial Sloan-Kettering Cancer Center.
A license and marketing agreement with AMAG Pharmaceuticals Inc' (formerly Advanced Magnetics), to market the functional molecular imaging agent Combidex®, was terminated in 2007.
Contact Information
MAIL
Cytogen Corporation
600 College Road East, CN5308
Princeton, NJ 08540-5308
USA
| |  | |
• View the DATABASE results for 'Cytogen Corporation' (2).
| | |
• View the NEWS results for 'Cytogen Corporation' (14).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |