 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Bo' was also found in the following services: | | | | |
|  |  |
| |
|
Rectal staging is necessary for the preoperative assessment of intra- and extramural tumor infiltration or the decision for adjuvant radio-chemotherapy.
One indication of MRI with luminal contrast enhancement is small bowel enteroclysis after duodenal intubation for visualization of inflammatory bowel wall thickening and other complications.
"Double contrast" enhancement of the bowel lumen is the administration of plain water or water with methylcellulose along with heavily T2 weighted sequences or contrast enhanced T1 weighted sequences.
Several oral contrast agents have been used for small bowel MRI: Mannitol, metamucil, locust bean gum, and PEG. All provide sufficient bowel distension and homogeneity, but suffer from side effects such as diarrhea. The volume of PEG or mannitol administered must be not too large in order to achieve the best compromise between distension and acceptance by the patient.
MR colonography with positive bowel lumen enhancement
requires higher concentrations of paramagnetic agents compared to the
available dedicated enteral contrast agents, IV compounds are used to dope water enemas for this purpose.
Some investigators advocate negative bowel enhancement
with Contrast Agents to suppress high signal bowel content in MRCP ( Magnetic resonance cholangiopancreaticography ).
The use of a mixture of metamucil and 20 ml of gadolinium chelate provides good homogeneity and good tolerance without diarrhea. | | | |  | |
• View the NEWS results for 'Gastrointestinal Imaging' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
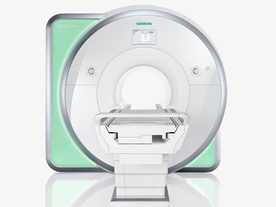
From Siemens Medical Systems;
Received FDA clearance in 2010.
The MAGNETOM Aera is a patient friendly, comfortable 1.5 Tesla MRI system with advanced radio frequency chain.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix + Day optimizing throughput), to enhance both productivity and image quality.
Tim 4G technology provides improved SNR. The standard system configuration of 48 radio frequency channels and 204 coil elements creates an imaging matrix that allows maximum use of coil elements at full field of view. Dot provides improved image consistency through new features like auto align, auto FoV and automatic bolus detection.
Device Information and Specification
CLINICAL APPLICATION
Whole body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder. Up to 60% more SNR with Tim 4G.
CHANNELS (min. / max. configuration)
48, 64
MINIMUM TE
3-D GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
3121 kg
DIMENSION H*W*D (gantry included)
145 x 231 x 219 cm
MAX. AMPLITUDE
33 or 45 mT/m
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 85 kVA
| |  | | | |
|  | |  |  |  |
| |
|
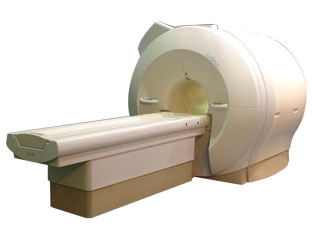
'Next generation MRI system 1.5T CHORUS developed by ISOL Technology is optimized for both clinical diagnostic imaging and for research development.
CHORUS offers the complete range of feature oriented advanced imaging techniques- for both clinical routine and research. The compact short bore magnet, the patient friendly design and the gradient technology make the innovation to new degree of perfection in magnetic resonance.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
Spin Echo, Gradient Echo, Fast Spin Echo,
Inversion Recovery ( STIR, Fluid Attenuated Inversion Recovery), FLASH, FISP, PSIF, Tur bo Flash ( MPRAGE ),TOF MR Angiography, Standard echo planar imaging package (SE-EPI, GE-EPI), Optional:
Advanced P.A. Imaging Package (up to 4 ch.), Advanced echo planar imaging package,
Single Shot and Diffusion Weighted EPI, IR/FLAIR EPI
STRENGTH
20 mT/m (Upto 27 mT/m)
| |  | |
• View the DATABASE results for 'CHORUS 1.5T™' (2).
| | | | |
|  |  | Searchterm 'Bo' was also found in the following services: | | | | |
|  |  |
| |
|
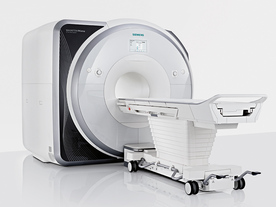
From Siemens Medical Systems;
Received FDA clearance in 2013.
The MAGNETOM Prisma is the 3T PowerPack for exploration that offers most demanding clinical and research challenges of today and the future. The latest parallel transmit technology, TimTX TrueShape, enables zooming into specific body regions for enhanced image quality. Furthermore, the Tim 4G integrated coil technology offers remarkable imaging flexibility and supports complex
examinations across the whole body.
Onsite upgrades to the MAGNETOM Prisma for customers who have already installed the 3 Tesla MAGNETOM Trio are possible.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
64, 128
MAGNET WEIGHT (gantry included)
13000 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground;
| |  | | | |
|  | |  |  |  |
| |
|
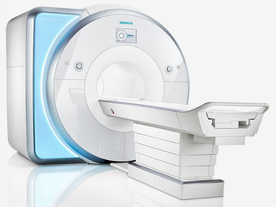
From Siemens Medical Systems;
Received FDA clearance in 2010.
MAGNETOM Skyra is a top-of-the-line, patient friendly wide bore 3 Tesla MRI system.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix and Day optimizing throughput), to enhance both productivity and image quality with the complete range of advanced applications for clinical routine and research. Tim 4G features lighter, trimmer MRI coils that take up less space inside the magnet but deliver a high coil element density with increased signal to noise ratio and the possibility to use high iPAT factors.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
48, 64, 128
Chemical shift imaging, single voxel spectroscopy
MINIMUM TE
3D T1 spoiled GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
5768 kg
DIMENSION H*W*D (gantry included)
173 x 231 x 219 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 110 kVA
| |  | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |