 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Device Information' found in 0 term [ ] and 70 definitions [ ] and 70 definitions [ ] ]
| previous 41 - 45 (of 70) nextResult Pages :  [1 2 3 4 5 6 7 8 9 10 11 12 13 14] [1 2 3 4 5 6 7 8 9 10 11 12 13 14] |  | |  | Searchterm 'Device Information' was also found in the following services: | | | | |
|  |  |
| |
|
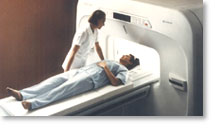
From Hitachi Medical Systems America, Inc.; because of its dependability, the MRP-7000™ remains popular more than a decade after the first U.S. system was shipped. This system maintains a high resale value, what has made it one of the most sought-after scanners on the used MRI equipment market.
Device Information and Specification CLINICAL APPLICATION Whole body DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP) SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOFIMAGING MODES Single, multislice, volume study horizontal 2.5 m x 2.1 m vertical | |  | | | |
|  | |  |  |  |
| |
|

From ONI Medical Systems, Inc.;
MSK-Extreme™ MRI system is a dedicated high field extremity imaging device, designed to provide orthopedic surgeons and other physicians with detailed diagnostic images of the foot, ankle, knee, hand, wrist and elbow, all with the clinical confidence and advantages derived from high field, whole body MRI units. The light weight (less than 650 kg) of the OrthOne System performs rapid patient studies, is easy to operate, has a patient friendly open environment and can be installed in a practice office or hospital, all at a cost similar to a low field extremity machine.
New features include a more powerful operating system that offers increased scan speed as well as a 160-mm knee coil with higher signal to noise ratio, and the option of a CD burner.
Device Information and Specification 16 cm knee, 18 cm lower extremity;; 12.3 cm upper extremity, additional high resolution v-SPEC Coils: 80 mm, 100 mm, or 145 mm. SE, FSE, GE2D, GE3D, Inversion recovery (IR), Driven Equilibrium, Fat Saturation (FS), STIR, MT, PD, Flow Compensation (FC), RF spoiling, MTE, No Phase Wrap (NPW) IMAGING MODES Scout, single, multislice, volume 2D less than 200 msec/image X/Y: 64-512; 2 pixel steps 4,096 grey lvls; 256 lvls in 3D POWER REQUIREMENTS 115VAC, 1phase, 20A; 208VAC, 3 phase, 30A COOLING SYSTEM TYPE LHe with 2 stage cold head 1.25m radial x 1.8m axial | |  | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|

From MagneVu;
The MagneVu 1000 is a compact, robust, and portable, permanent magnet MRI system and operates without special shielding or costly site preparation.
This MRI device utilizes a patented non-homogeneous magnetic field image acquisition method to achieve high performance imaging. The MagneVu 1000 MRI scanner is designed for MRI of the extremities with the current specialty areas in diabetes and rheumatoid arthritis. Easy access is afforded for claustrophobic, pediatric, or limited mobility patients. In August 1998
FDA marketing clearance and other regulatory approvals have been received. Until 2008, over 130 devices in the US are in use. Some further developments of MagneVu's extremity scanner are: 'truly Plug n' Play MRI™' and iSiS ( which adds wireless capability to the second generation MV1000-XL).
Device Information and Specification IMAGING MODES 3-dimensional multi-echo data acquisition | |  | |
• View the DATABASE results for 'MagneVu 1000' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Device Information' was also found in the following services: | | | | |
|  |  |
| |
|
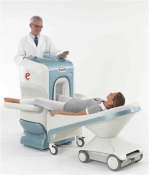
O-scan is manufactured and distributed by Esaote SpA
O-scan is a compact, dedicated extremity MRI system designed for easy installation and high throughput. The complete system fits in a 9' x 10' room, doesn't need for RF or magnetic shielding and it plugs in the wall. The 0.31T permanent magnet along with dual phased array RF coils, and advanced imaging protocols provide outstanding image quality and fast 25 minute complete examinations.
Esaote North America is the exclusive distributor of the O-scan system in the USA.
Device Information and Specification CLINICAL APPLICATION Dedicated Extremity
PULSE SEQUENCES
SE, HSE, HFE, GE, 2dGE, ME, IR, STIR, Stir T2, GESTIR, TSE, TME, FSE STIR, FSE ( T1, T2), X-Bone, Turbo 3DT1, 3D SHARC, 3D SST1, 3D SST2 2D: 2mm - 10 mm, 3D: 0.6 - 10 mm POWER REQUIREMENTS 100/110/200/220/230/240 | |  | | | |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | |
• View the DATABASE results for 'OPART™' (2).
| | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |