 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'High Field MRI' found in 1 term [ ] and 16 definitions [ ] and 16 definitions [ ], (+ 18 Boolean[ ], (+ 18 Boolean[ ] results ] results
| previous 16 - 20 (of 35) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4]  [5 6 7] [5 6 7] |  | |  | Searchterm 'High Field MRI' was also found in the following services: | | | | |
|  |  |
| |
|
Quick Overview
DESCRIPTION
Localized inhomogeneous brightness
HELP
Check the correct positioning, call the service
Uneven intensity or brightness may occasionally be noted on high field MRI e.g. of the brain. There are various causes of localized inhomogeneous brightness across the MRI images such as improper tuning of the RF transceiver, unbalanced deposition of the RF energy due to incorrect geometry and localized attenuation due to positioning or anatomical variants.

Image Guidance
Tuning of the RF transceiver, a homogeneity correction filter and better positioning of the scanned object and/or coil in the scanner. | |  | | | |
|  | |  |  |  |
| |
|
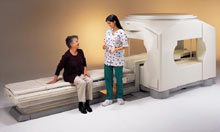
From Toshiba America Medical Systems Inc.;
the Ultra™ system was developed to help healthcare providers be more competitive by delivering greater patient comfort and a broad range of clinical capabilities, says Anita Bowler, product manager, MRI Business Unit, Toshiba America Medical Systems Inc With its unique, powerful gradient technology, the Ultra™ performs advanced clinical studies and consistently provides high-resolution images that are typically associated with high field MRI systems. At the same time, the Ultra™ offers a truly open feeling that makes patients more relaxed, especially those with claustrophobic tendencies.
Device Information and Specification CLINICAL APPLICATION Whole Body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI, Single shot EPI diffusion, True SSFP, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC, Black Blood MRA POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | | | |
|  | |  |  |  |
| |
|
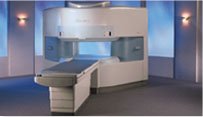
From Hitachi Medical Systems America, Inc.;
the AIRIS made its debut in 1995. Hitachi followed up with the AIRIS II system, which has proven equally successfully. 'All told, Hitachi has installed more than 1,000 MRI systems in the U.S., holding more than 17 percent of the total U.S. MRI installed base, and more than half of the installed base of open MR systems,' says Antonio Garcia, Frost and Sullivan industry research analyst.
Now Altaire employs a blend of innovative Hitachi features called VOSIā¢ technology, optimizing each sub-system's performance in concert with the
other sub-systems, to give the seamless mix of high- field performance
and the patient comfort, especially for claustrophobic patients, of open MR systems.
Device Information and Specification
CLINICAL APPLICATION
Whole body
DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP)
SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 3.6 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 8 - 250msec IR: 5.2 -7,680msec GE: 1.8 - 2,000 msec FSE: 5.2 - 7,680
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
COOLING SYSTEM TYPE
Water-cooled
3.1 m lateral, 3.6 m vertical
| |  | |
• View the DATABASE results for 'Altaire™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'High Field MRI' was also found in the following services: | | | | |
|  |  |
| |
|
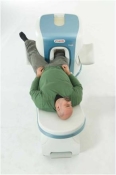
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
Opt. (WIP) Single and Multi Voxel
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 1.2 msec min. FE
less than 0.015 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
4050 kg (bare magnet incl. L-He)
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart AG™ with Pianissimo' (2).
| | | | |
|  | |  |  |  |
| |
|
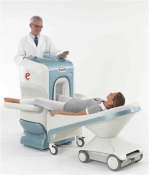
O-scan is manufactured and distributed by Esaote SpA
O-scan is a compact, dedicated extremity MRI system designed for easy installation and high throughput. The complete system fits in a 9' x 10' room, doesn't need for RF or magnetic shielding and it plugs in the wall. The 0.31T permanent magnet along with dual phased array RF coils, and advanced imaging protocols provide outstanding image quality and fast 25 minute complete examinations.
Esaote North America is the exclusive distributor of the O-scan system in the USA.
Device Information and Specification CLINICAL APPLICATION Dedicated Extremity
PULSE SEQUENCES
SE, HSE, HFE, GE, 2dGE, ME, IR, STIR, Stir T2, GESTIR, TSE, TME, FSE STIR, FSE ( T1, T2), X-Bone, Turbo 3DT1, 3D SHARC, 3D SST1, 3D SST2 2D: 2mm - 10 mm, 3D: 0.6 - 10 mm POWER REQUIREMENTS 100/110/200/220/230/240 | |  | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |