 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Low Field MRI' found in 1 term [ ] and 7 definitions [ ] and 7 definitions [ ], (+ 17 Boolean[ ], (+ 17 Boolean[ ] results ] results
| previous 11 - 15 (of 25) nextResult Pages :  [1] [1]  [2] [2]  [3 4 5] [3 4 5] |  | |  | Searchterm 'Low Field MRI' was also found in the following services: | | | | |
|  |  |
| |
|
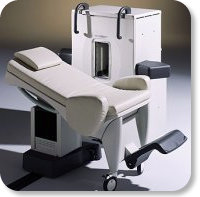
Developed by GE Lunar; the ARTOSCAN™-M is designed specifically for in-office musculoskeletal imaging. ARTOSCAN-M's compact, modular design al lows placing within a clinical environment, bringing MRI to the patient. Patients remain outside the magnet at all times during the examinations, enabling constant patient-technologist contact. ARTOSCAN-M requires no special RF room, magnetic shielding, special power supply or air conditioning.
The C-SCAN™ (also known as Artoscan C) is developed from the ARTOSCAN™ - M, with a new computer platform.
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
SLICE THICKNESS
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240V
| |  | | | | | | | | |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
SE, IR, FSE, FIR, GE, SG, BASG, PBSG, PCIR, DWI, Radial, Angiography: TOF, FLUTE (Fluoro-triggered bolus MRA), Time-resolved MRA
IMAGING MODES
Single, multislice, volume study
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
| |  | |
• View the DATABASE results for 'Echelon™ 1.5T' (2).
| | |
• View the NEWS results for 'Echelon™ 1.5T' (3).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|

From ONI Medical Systems, Inc.;
MSK-Extreme™ MRI system is a dedicated high field extremity imaging device, designed to provide orthopedic surgeons and other physicians with detailed diagnostic images of the foot, ankle, knee, hand, wrist and elbow, all with the clinical confidence and advantages derived from high field, whole body MRI units. The light weight (less than 650 kg) of the OrthOne System performs rapid patient studies, is easy to operate, has a patient friendly open environment and can be installed in a practice office or hospital, all at a cost similar to a low field extremity machine.
New features include a more powerful operating system that offers increased scan speed as well as a 160-mm knee coil with higher signal to noise ratio, and the option of a CD burner.
Device Information and Specification 16 cm knee, 18 cm lower extremity;; 12.3 cm upper extremity, additional high resolution v-SPEC Coils: 80 mm, 100 mm, or 145 mm. SE, FSE, GE2D, GE3D, Inversion recovery (IR), Driven Equilibrium, Fat Saturation (FS), STIR, MT, PD, Flow Compensation (FC), RF spoiling, MTE, No Phase Wrap (NPW) IMAGING MODES Scout, single, multislice, volume 2D less than 200 msec/image X/Y: 64-512; 2 pixel steps 4,096 grey lvls; 256 lvls in 3D POWER REQUIREMENTS 115VAC, 1phase, 20A; 208VAC, 3 phase, 30A COOLING SYSTEM TYPE LHe with 2 stage cold head 1.25m radial x 1.8m axial | |  | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | Searchterm 'Low Field MRI' was also found in the following services: | | | | |
|  |  |
| |
|
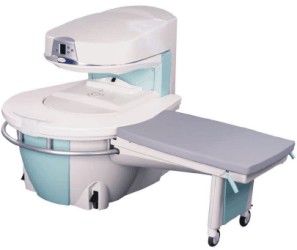
Manufactured by Esaote S.p.A.;
a low field open MRI scanner with permanent magnet for orthopedic use. The outstanding feature of this MRI system is a patient friendly design with 24 cm diameter, which al lows the imaging of extremities and small body parts like shoulder MRI. The power consumption is around 1.3 kW and the needed minimum floor space is an area of 16 sq m.
At RSNA 2006 Hologic Inc. introduced a new dedicated extremity MRI scanner, the Opera. Manufactured by Esaote is the Opera a redesign of Esaote's 0.2 Tesla E-Scan XQ platform, which now enables complete imaging of all extremities, including hip and shoulder applications. 'Real-time positioning' reportedly speeds patient setup and reduces exam times.
Esaote North America and Hologic Inc are the U.S. distributors of this MRI device.
Device Information and Specification CLINICAL APPLICATION Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE IMAGING MODES Single, multislice, volume study, fast scan, multi slab2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm 4096 gray lvls, 256 lvls in 3D POWER REQUIREMENTS 2,0 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'Opera (E-SCAN™ XQ)' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|

If a device is to be labeled MR Safe, the fol lowing information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment (i.e. the MRI scan room) it does not pose an increased safety risk to the patient or other personnel,
•
a scientifically-based rationale for why data are not necessary to prove the safety of the device in the MR environment (for example, a passive device made entirely of a polymer known to be nonreactive in strong magnetic fields).
If a device is to be labeled MR Compatible, the fol lowing information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment, it is MR safe that it performs its intended function without performance degradation, and that it does not adversely affect the function of the MRI scanner (e.g. no significant image artifacts or noise). Any image artifact or noise due to the medical device should be quantified (e.g., % volume affected, signal to noise ratio),
•
a scientifically-based rationale for why data are not necessary to prove the compatibility of the device in the MRI environment.
Test Conditions:
The static magnetic field strength ( Gauss (G) or Tesla (T)) to which the device was tested and demonstrated to be MRI 'safe', 'compatible', or 'intended for use in' should be related to typical machine ratings (e.g. 0.5 T, 1.5 T, 2.0 T, and shielded or unshielded magnet, etc).
The same conditions should be used for the spatial gradient ( field strength per unit distance (i.e., G/cm)) in which the device was tested and demonstrated to be 'safe', 'compatible', or 'intended for use in'.
Also the RF transmitter power used during testing of the device, should be related to this typical machine ratings. | |  | |
• View the DATABASE results for 'MR Compatibility' (4).
| | |
• View the NEWS results for 'MR Compatibility' (2).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |