 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Low Field MRI' found in 1 term [ ] and 7 definitions [ ] and 7 definitions [ ], (+ 17 Boolean[ ], (+ 17 Boolean[ ] results ] results
| previous 16 - 20 (of 25) nextResult Pages :  [1] [1]  [2] [2]  [3 4 5] [3 4 5] |  | |  | Searchterm 'Low Field MRI' was also found in the following services: | | | | |
|  |  |
| |
|
The owner of MRI equipment has to ensure that the equipment does fulfill the local requirements.
In some countries, the requirements are more stringent than in others; in other countries, they are nonexistent.
The user in general is unable to check power output, gradient strength, or even field strength.
The manufacturer has to cover authorized hardware and software updates after the initial installation and has to give guarantee for the requirements.
Specially designed computer programs usually supervise the power output of MRI devices and will not al low or will interrupt any imaging or spectroscopy procedure exceeding those limits considered safe.
See also European Medicines Agency, FDA information:
www.fda.gov/cdrh/safety/mrisafety.html | |  | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|
•
In the 1930's, Isidor Isaac Rabi (Columbia University) succeeded in detecting and measuring single states of rotation of atoms and molecules, and in determining the mechanical and magnetic moments of the nuclei.
•
Felix Bloch (Stanford University) and Edward Purcell (Harvard University) developed instruments, which could measure the magnetic resonance in bulk material such as liquids and solids. (Both honored with the Nobel Prize for Physics in 1952.) [The birth of the NMR spectroscopy]
•
In the early 70's, Raymond Damadian (State University of New York) demonstrated with his NMR device, that there are different T1 relaxation times between normal and abnormal tissues of the same type, as well as between different types of normal tissues.
•
In 1973, Paul Lauterbur (State University of New York) described a new imaging technique that he termed Zeugmatography. By utilizing gradients in the magnetic field, this technique was able to produce a two-dimensional image (back-projection). (Through analysis of the characteristics of the emitted radio waves, their origin could be determined.) Peter Mans field further developed the utilization of gradients in the magnetic field and the mathematically analysis of these signals for a more useful imaging technique. (Paul C Lauterbur and Peter Mans field were awarded with the 2003 Nobel Prize in Medicine.)
•
1977/78: First images could be presented.
A cross section through a finger by Peter Mansfield and Andrew A. Maudsley.
Peter Mansfield also could present the first image through the abdomen.
•
In 1977, Raymond Damadian completed (after 7 years) the first MR scanner (Indomitable). In 1978, he founded the FONAR Corporation, which manufactured the first commercial MRI scanner in 1980. Fonar went public in 1981.
•
1981: Schering submitted a patent application for Gd-DTPA dimeglumine.
•
1982: The first 'magnetization-transfer' imaging by Robert N. Muller.
•
In 1983, Toshiba obtained approval from the Ministry of Health and Welfare in Japan for the first commercial MRI system.
•
1986: Jürgen Hennig, A. Nauerth, and Hartmut Friedburg (University of Freiburg) introduced RARE (rapid acquisition with relaxation enhancement) imaging. Axel Haase, Jens Frahm, Dieter Matthaei, Wolfgang Haenicke, and Dietmar K. Merboldt (Max-Planck-Institute, Göttingen) developed the FLASH ( fast low angle shot) sequence.
•
1988: Schering's MAGNEVIST gets its first approval by the FDA.
•
In 1991, fMRI was developed independently by the University of Minnesota's Center for Magnetic Resonance Research (CMRR) and Massachusetts General Hospital's (MGH) MR Center.
•
From 1992 to 1997 Fonar was paid for the infringement of it's patents from 'nearly every one of its competitors in the MRI industry including giant multi-nationals as Toshiba, Siemens, Shimadzu, Philips and GE'.
| | | |  | |
• View the DATABASE results for 'MRI History' (6).
| | |
• View the NEWS results for 'MRI History' (1).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
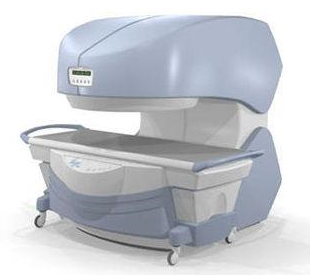
From
Millennium Technology Inc.
This open C-shaped MRI system eases patient comfort and technologist maneuverability. This low cost scanner is build for a wide range of applications. The Virgo™ patient table is detachable and moves on easy rolling castors. Able to accommodate patient weights up to 160 kg, the tabletop has a range of motion of 30 cm in the lateral direction and 90cm in the longitudinal direction. Images generated with this scanner can only be viewed (without data loss) on Millennium's proprietary viewing software.
Device Information and Specification CLINICAL APPLICATION Whole body Head, Body, Neck, Knee, Shoulder,
Spine, Wrist, Breast, Extremity, Lumbar Spine, TMJ
IMAGING MODES Localizer, single slice, multislice, volume, fast, POMP, multi slab, cine, slice and frequency zip, extended dynamic range, tailored RF | |  | | | |
|  |  | Searchterm 'Low Field MRI' was also found in the following services: | | | | |
|  |  |
| |
|
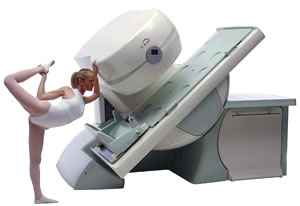
From Esaote S.p.A.;
Esaote introduced the new G-SCAN at the RSNA in Dec. 2004. The G-SCAN covers almost all musculoskeletal applications including the spine. The tilting gantry is designed for scanning in weight-bearing positions. This unique MRI scanner is developed in line with the Esaote philosophy of creating high quality MRI systems that are easy to install and that have a low breakeven point.
Device Information and Specification
SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
100 up to 350 mm, 25 mm displayed
POWER REQUIREMENTS
100/110/200/220/230/240 V
| |  | |
• View the DATABASE results for 'G-SCAN' (3).
| | | | |
|  | |  |  |  |
| |
|

From GE Healthcare;
GE Healthcare has added the Signa HDe 1.5T™, a compact MRI device at an affordable price to its family of MRI products. It has a single electronic cabinet that can be positioned inside the scanner room rather than
in a separate equipment room. The Signa HDe 1.5T can be installed in the same physical location as 0.5T MRI systems with minimal construction costs. According to GE, the installation has been simplified to last only 7 days and has a 30 percent smaller footprint than a typical 1.5T system.
The 1.5T Signa™ HDe MRI system is substantially equivalent to the currently marketed GE 1.5T machines. The data acquisition system supports 1, 4, 8 independent receive channels and multiple independent coil elements per channel during a single acquisition series. The gradient specifications of HDe are lower than other GE Signa 1.5T MRI systems, but it can support clinical applications in cardiac and spectroscopy imaging.
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Compact short bore 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 less than 0.03 L/hr liquid helium | |  | |
• View the NEWS results for 'Signa HDe 1.5Tâ„¢' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |