 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | 'Multi Echo Pulse Sequence' | |
Result : Searchterm 'Multi Echo Pulse Sequence' found in 1 term [ ] and 1 definition [ ] and 1 definition [ ], (+ 18 Boolean[ ], (+ 18 Boolean[ ] results ] results
| previous 6 - 10 (of 20) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |  | | |  |  |  |
| |
|
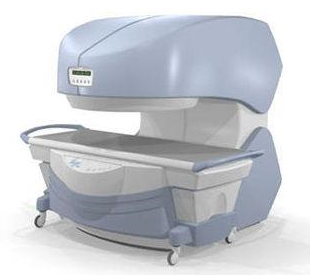
From
Millennium Technology Inc.
This open C-shaped MRI system eases patient comfort and technologist maneuverability. This low cost scanner is build for a wide range of applications. The Virgo™ patient table is detachable and moves on easy rolling castors. Able to accommodate patient weights up to 160 kg, the tabletop has a range of motion of 30 cm in the lateral direction and 90cm in the longitudinal direction. Images generated with this scanner can only be viewed (without data loss) on Millennium's proprietary viewing software.
Device Information and Specification CLINICAL APPLICATION Whole body Head, Body, Neck, Knee, Shoulder,
Spine, Wrist, Breast, Extremity, Lumbar Spine, TMJ
IMAGING MODES Localizer, single slice, multislice, volume, fast, POMP, multi slab, cine, slice and frequency zip, extended dynamic range, tailored RF | |  | | | |
|  | |  |  |  |
| |
|

From GE Healthcare;
'EXCITE technology has the potential to open the door to new imaging techniques and clinical applications, leaping beyond conventional two and three-dimensional MRI to true 4D imaging that will improve the diagnosis of disease throughout the human body from head to foot.' Robert R. Edelman, M.D., Professor of Radiology at Northwestern University Medical School and Chairman, Department of Radiology, at Evanston Northwestern Healthcare.
Device Information and Specification CLINICAL APPLICATION Whole body Head and body coil standard; all other coils optional; open architecture makes system compatible with a wide selection of coils Optional 2D/3D brain and prostate Standard: SE, IR, 2D/3D GRE and SPGR, Angiography: 2D/3D TOF, 2D/3D Phase Contrast;; 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, FGRET, Spiral, TensorTR 1.3 to 12000 msec in increments of 1 msec TE 0.4 to 2000 msec in increments of 1 msec 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode 0.08 mm; 0.02 mm optional POWER REQUIREMENTS 480 or 380/415 less than 0.03 L/hr liquid heliumSTRENGTH SmartSpeed 23 mT/m, HiSpeed Plus 33 mT/m, EchoSpeed Plus 33 mT/m 4.0 m x 2.8 m axial x radial | |  | |
• View the DATABASE results for 'Signa Infinity 1.5T™ with Excite' (2).
| | | | |
|  | |  |  |  |
| |
|
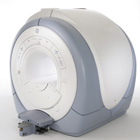
From GE Healthcare;
The Signa HDx MRI system is GE's leading edge whole body magnetic resonance scanner designed to support high resolution, high signal to noise ratio, and short scan times.
Signa HDx 3.0T offers new technologies like ultra-fast image reconstruction through the new XVRE recon engine, advancements in parallel imaging algorithms and the broadest range of premium applications. The HD applications, PROPELLER (high-quality brain imaging extremely resistant to motion artifacts), TRICKS (contrast-enhanced angiographic vascular lower leg imaging), VIBRANT (for breast MRI), LAVA (high resolution liver imaging with shorter breath holds and better organ coverage) and MR Echo (high-definition cardiac images in real time) offer unique capabilities.
Device Information and Specification CLINICAL APPLICATION Whole body
CONFIGURATION Compact short bore SE, IR, 2D/3D GRE, RF-spoiled GRE, 2DFGRE, 2DFSPGR, 3DFGRE, 3DFSPGR, 3DTOFGRE, 3DFSPGR, 2DFSE, 2DFSE-XL, 2DFSE-IR, T1-FLAIR, SSFSE, EPI, DW-EPI, BRAVO, Angiography: 2D/3D TOF, 2D/3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer H*W*D 240 x 2216,6 x 201,6 cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. | |  | | | |
|  | |  |  |  |
| |
|
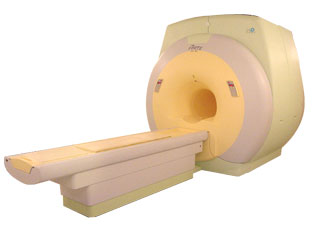
From ISOL Technology
'Ultra high field MR system, it's right close to you.
FORTE 3.0T is the new standard for the future ultra high field MR system.
If you are pushing the limits of your existing clinical MR scanner, the FORTE will surely take you to the next level of diagnostic imaging.
FORTE is the core leader of the medical technology in the 21st century. Proving effects of fMRI that cannot be measured with MRI less than 2.0T.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
128 x 128, 256 x 256, 512 x 512, 1024 x 1024
| |  | |
• View the DATABASE results for 'FORTE 3.0T™' (2).
| | | | |
|  | |  |  |  |
| |
|

From MagneVu;
The MagneVu 1000 is a compact, robust, and portable, permanent magnet MRI system and operates without special shielding or costly site preparation.
This MRI device utilizes a patented non-homogeneous magnetic field image acquisition method to achieve high performance imaging. The MagneVu 1000 MRI scanner is designed for MRI of the extremities with the current specialty areas in diabetes and rheumatoid arthritis. Easy access is afforded for claustrophobic, pediatric, or limited mobility patients. In August 1998
FDA marketing clearance and other regulatory approvals have been received. Until 2008, over 130 devices in the US are in use. Some further developments of MagneVu's extremity scanner are: 'truly Plug n' Play MRI™' and iSiS ( which adds wireless capability to the second generation MV1000-XL).
Device Information and Specification IMAGING MODES 3-dimensional multi-echo data acquisition | |  | |
• View the DATABASE results for 'MagneVu 1000' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |