 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Multi Phase Imaging' found in 1 term [ ] and 0 definition [ ] and 0 definition [ ], (+ 19 Boolean[ ], (+ 19 Boolean[ ] results ] results
| previous 6 - 10 (of 20) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |  | |  | Searchterm 'Multi Phase Imaging' was also found in the following service: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
IMAGING MODES
Single, multislice, volume study, fast scan, multi slab
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240
| |  | | | |
|  | |  |  |  |
| |
|
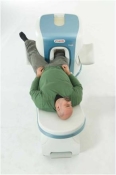
O-scan is manufactured and distributed by Esaote SpA
O-scan is a compact, dedicated extremity MRI system designed for easy installation and high throughput. The complete system fits in a 9' x 10' room, doesn't need for RF or magnetic shielding and it plugs in the wall. The 0.31T permanent magnet along with dual phased array RF coils, and advanced imaging protocols provide outstanding image quality and fast 25 minute complete examinations.
Esaote North America is the exclusive distributor of the O-scan system in the USA.
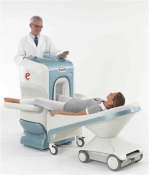
Device Information and Specification CLINICAL APPLICATION Dedicated Extremity
PULSE SEQUENCES
SE, HSE, HFE, GE, 2dGE, ME, IR, STIR, Stir T2, GESTIR, TSE, TME, FSE STIR, FSE ( T1, T2), X-Bone, Turbo 3DT1, 3D SHARC, 3D SST1, 3D SST2 2D: 2mm - 10 mm, 3D: 0.6 - 10 mm POWER REQUIREMENTS 100/110/200/220/230/240 | |  | | | |
|  | |  |  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
(July 20, 2009 - EPIX Pharmaceuticals, Inc. announced today that, in light of the company's lack of capital and inability to obtain additional financing or consummate a strategic transaction, it has entered into an Assignment for the Benefit of Creditors, effective immediately, in accordance with Massachusetts law).
EPIX has been a specialty pharmaceutical firm developing targeted contrast agents to improve the capability of MRI as a diagnostic tool for a variety of diseases. Gadofosveset trisodium (formerly MS-325, Vasovist™, now ABLAVARt™), is an injectable intravascular contrast agents designed for multiple vascular imaging applications, including peripheral vascular disease and coronary artery disease.
EPIX conducted a pivotal Phase III trial for the detection of peripheral vascular disease, as well as a Phase II feasibility trial for coronary artery disease diagnosis.
To ensure rapid development and adoption of gadofosveset trisodium into clinical practice upon regulatory approval, EPIX pursued an aggressive product development plan and commercialization strategy. The Company established an exclusive, worldwide sales and marketing agreement with Bayer Schering Pharma AG. EPIX also established corporate collaborations with GE Healthcare, Philips Medical Systems and Siemens Medical Systems, the three leading MRI manufacturers, which together account for approximately 80 percent of the MRI machines installed worldwide.
EPIX had other MRI contrast agents under development, most significantly a novel prototype blood clot agent ( EP-2104R). Potential clinical applications for this type of agent include detection of deep venous thrombosis, pulmonary embolism and blood clots in the coronary and carotid arteries. Currently, there is no high resolution imaging technique to directly visualize blood clots in patients with suspected cardiovascular disease.
| |  | |
• View the DATABASE results for 'EPIX Pharmaceuticals, Inc.' (7).
| | |
• View the NEWS results for 'EPIX Pharmaceuticals, Inc.' (69).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | Searchterm 'Multi Phase Imaging' was also found in the following service: | | | | |
|  |  |
| |
|
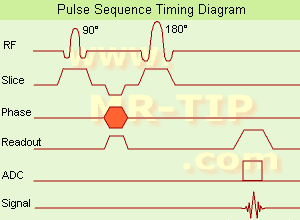
(SE) The most common pulse sequence used in MR imaging is based of the detection of a spin or Hahn echo. It uses 90° radio frequency pulses to excite the magnetization and one or more 180° pulses to refocus the spins to generate signal echoes named spin echoes (SE).
In the pulse sequence timing diagram, the simplest form of a spin echo sequence is illustrated.
The 90° excitation pulse rotates the longitudinal magnetization ( Mz) into the xy-plane and the dephasing of the transverse magnetization (Mxy) starts.
The following application of a 180° refocusing pulse (rotates the magnetization in the x-plane) generates signal echoes. The purpose of the 180° pulse is to re phase the spins, causing them to regain coherence and thereby to recover transverse magnetization, producing a spin echo.
The recovery of the z-magnetization occurs with the T1 relaxation time and typically at a much slower rate than the T2-decay, because in general T1 is greater than T2 for living tissues and is in the range of 100-2000 ms.
The SE pulse sequence was devised in the early days of NMR days by Carr and Purcell and exists now in many forms: the multi echo pulse sequence using single or multislice acquisition, the fast spin echo (FSE/TSE) pulse sequence, echo planar imaging (EPI) pulse sequence and the gradient and spin echo (GRASE) pulse sequence;; all are basically spin echo sequences.
In the simplest form of SE imaging, the pulse sequence has to be repeated as many times as the image has lines. Contrast values:
PD weighted: Short TE (20 ms) and long TR.
T1 weighted: Short TE (10-20 ms) and short TR (300-600 ms)
T2 weighted: Long TE (greater than 60 ms) and long TR (greater than 1600 ms)
With spin echo imaging no T2* occurs, caused by the 180° refocusing pulse. For this reason, spin echo sequences are more robust against e.g., susceptibility artifacts than gradient echo sequences.
See also Pulse Sequence Timing Diagram to find a description of the components.
| | | |  | |
• View the DATABASE results for 'Spin Echo Sequence' (24).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
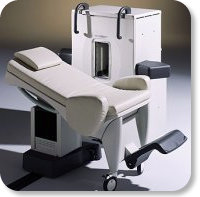
Developed by GE Lunar; the ARTOSCAN™-M is designed specifically for in-office musculoskeletal imaging. ARTOSCAN-M's compact, modular design allows placing within a clinical environment, bringing MRI to the patient. Patients remain outside the magnet at all times during the examinations, enabling constant patient-technologist contact. ARTOSCAN-M requires no special RF room, magnetic shielding, special power supply or air conditioning.
The C-SCAN™ (also known as Artoscan C) is developed from the ARTOSCAN™ - M, with a new computer platform.
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
SLICE THICKNESS
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240V
| |  | |
• View the DATABASE results for 'ARTOSCAN™ - M' (3).
| | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |