 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Passive Device' found in 1 term [ ] and 1 definition [ ] and 1 definition [ ], (+ 18 Boolean[ ], (+ 18 Boolean[ ] results ] results
| 1 - 5 (of 20) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |  | |  | Searchterm 'Passive Device' was also found in the following service: | | | | |
|  |  |
| |
|
The term 'passive' refers to any medical device that serves its function without the supply of power. Examples of passive devices include but are not limited to aneurysm clips, shunts, scalpels, IV poles, and oxygen bottles. | |  | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | | | | • Share the entry 'Passive Device':    | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|

If a device is to be labeled MR Safe, the following information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment (i.e. the MRI scan room) it does not pose an increased safety risk to the patient or other personnel,
•
a scientifically-based rationale for why data are not necessary to prove the safety of the device in the MR environment (for example, a passive device made entirely of a polymer known to be nonreactive in strong magnetic fields).
If a device is to be labeled MR Compatible, the following information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment, it is MR safe that it performs its intended function without performance degradation, and that it does not adversely affect the function of the MRI scanner (e.g. no significant image artifacts or noise). Any image artifact or noise due to the medical device should be quantified (e.g., % volume affected, signal to noise ratio),
•
a scientifically-based rationale for why data are not necessary to prove the compatibility of the device in the MRI environment.
Test Conditions:
The static magnetic field strength ( Gauss (G) or Tesla (T)) to which the device was tested and demonstrated to be MRI 'safe', 'compatible', or 'intended for use in' should be related to typical machine ratings (e.g. 0.5 T, 1.5 T, 2.0 T, and shielded or unshielded magnet, etc).
The same conditions should be used for the spatial gradient ( field strength per unit distance (i.e., G/cm)) in which the device was tested and demonstrated to be 'safe', 'compatible', or 'intended for use in'.
Also the RF transmitter power used during testing of the device, should be related to this typical machine ratings. | |  | |
• View the DATABASE results for 'MR Compatibility' (4).
| | |
• View the NEWS results for 'MR Compatibility' (2).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
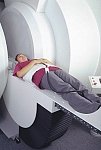
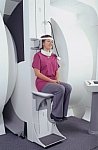
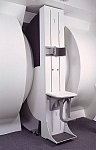
From FONAR Corporation;
in October of 2004, the company changed the product name of the Stand-Up™ MRI to the Upright™ MRI. The Indomitable™, Upright™ MRI is the only open MRI in the world that can perform positional MRI (pMRI), i.e. the Upright MRI™ scans patients in upright, weight-bearing positions, in addition to the conventional lie-down positions. The Upright™ MRI is the only device that can scan patients in the position their symptoms occur, in their position of pain. In early clinical reports independently confirm the effectiveness and potential of positional MRI. In October 2000, Fonar received permission to market the Indomitable™ from the FDA.
Device Information and Specification CLINICAL APPLICATION Whole body -
weight-bearing MRI -
position imaging (flexion, extension, bending, standing, sitting and recumbent scanning)
CONFIGURATION Front-open and Top-open MRIPOWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Water, closed-loop | |  | |
• View the DATABASE results for 'Upright™ MRI' (5).
| | |
• View the NEWS results for 'Upright™ MRI' (9).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Passive Device' was also found in the following service: | | | | |
|  |  |
| |
|
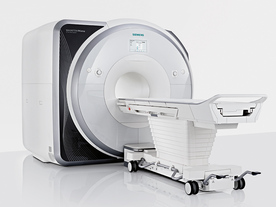
From Siemens Medical Systems;
Received FDA clearance in 2013.
The MAGNETOM Prisma is the 3T PowerPack for exploration that offers most demanding clinical and research challenges of today and the future. The latest parallel transmit technology, TimTX TrueShape, enables zooming into specific body regions for enhanced image quality. Furthermore, the Tim 4G integrated coil technology offers remarkable imaging flexibility and supports complex
examinations across the whole body.
Onsite upgrades to the MAGNETOM Prisma for customers who have already installed the 3 Tesla MAGNETOM Trio are possible.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
64, 128
MAGNET WEIGHT (gantry included)
13000 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground;
| |  | | | |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
Opt. (WIP) Single and Multi Voxel
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 1.2 msec min. FE
less than 0.015 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
4050 kg (bare magnet incl. L-He)
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart AG™ with Pianissimo' (2).
| | | | |
|  | |  |  |
|  | 1 - 5 (of 20) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |
| |
|
| |
 | Look
Ups |
| |