 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Solenoid Coil' found in 1 term [ ] and 0 definition [ ] and 0 definition [ ], (+ 13 Boolean[ ], (+ 13 Boolean[ ] results ] results
| 1 - 5 (of 14) nextResult Pages :  [1] [1]  [2 3] [2 3] |  | | |  |  |  |
| Solenoid Coil |   |
| |
|
A coil of wire wound in the form of a long cylinder. When a current is passed through the coil, the magnetic field within the coil is relatively uniform. Solenoid RF coils are commonly used when the static magnetic field is perpendicular to the long axis of the body.
The reason against a solenoidal coil is that the signal is missed when the axis of the solenoidal coil is coaxial with the horizontal magnetic field Bo. If the coil's axis can be put near a right angle (60° to 90°) with the principal field, so the loss in the signal is greatly dismissed, and good SNR can be achieved.
The two forms are single turn solenoid and multiple turn solenoids. | |  | | | | • Share the entry 'Solenoid Coil':    | | | | |
|  | |  |  |  |
| |
|
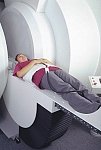
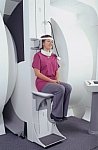
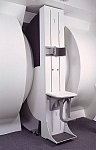
From FONAR Corporation;
in October of 2004, the company changed the product name of the Stand-Up™ MRI to the Upright™ MRI. The Indomitable™, Upright™ MRI is the only open MRI in the world that can perform positional MRI (pMRI), i.e. the Upright MRI™ scans patients in upright, weight-bearing positions, in addition to the conventional lie-down positions. The Upright™ MRI is the only device that can scan patients in the position their symptoms occur, in their position of pain. In early clinical reports independently confirm the effectiveness and potential of positional MRI. In October 2000, Fonar received permission to market the Indomitable™ from the FDA.
Device Information and Specification CLINICAL APPLICATION Whole body -
weight-bearing MRI -
position imaging (flexion, extension, bending, standing, sitting and recumbent scanning)
CONFIGURATION Front-open and Top-open MRIPOWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Water, closed-loop | |  | |
• View the DATABASE results for 'Upright™ MRI' (5).
| | |
• View the NEWS results for 'Upright™ MRI' (9).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | |
• View the DATABASE results for 'OPART™' (2).
| | | | |
|  | |  |  |  |
| |
|

From Philips Medical Systems;
this active shielded member of the Panorama product line combines the advantages of one 1.0 T system's with the possibilities of an open MRI system. The open design helps ease anxiety for claustrophobic patients and increased patient comfort whereby the field strength provides spectacular image quality and fast patient throughput.
Device Information and Specification CLINICAL APPLICATION Whole body Vertically opposed solenoids, head, head-neck, extremity, neck, body/ spine M-XL, shoulder, bilateral breast, wrist, TMJ, flex XS-S-M-L-XL-XXL SE, FE, IR, STIR, FFE, DEFFE, DESE, TSE, DETSE, Single shot SE, DRIVE, Balanced FFE, MRCP, FLAIR, Turbo FLAIR, IR-TSE, T1-STIR TSE, T2-STIR TSE, Diffusion Imaging, 3D SE, 3D FFE, Contrast Perfusion Analysis, MTC;; Angiography: CE-ANGIO, MRA 2D, 3D TOFOpen x 47 cm x infinite (side-first patient entry) POWER REQUIREMENTS 400/480 V | |  | |
• View the DATABASE results for 'Panorama 1.0T™' (2).
| | | | |
|  | |  |  |  |
| |
|
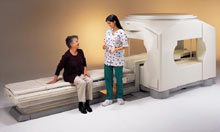
From Toshiba America Medical Systems Inc.;
the Ultra™ system was developed to help healthcare providers be more competitive by delivering greater patient comfort and a broad range of clinical capabilities, says Anita Bowler, product manager, MRI Business Unit, Toshiba America Medical Systems Inc With its unique, powerful gradient technology, the Ultra™ performs advanced clinical studies and consistently provides high-resolution images that are typically associated with high field MRI systems. At the same time, the Ultra™ offers a truly open feeling that makes patients more relaxed, especially those with claustrophobic tendencies.
Device Information and Specification CLINICAL APPLICATION Whole Body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI, Single shot EPI diffusion, True SSFP, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC, Black Blood MRA POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | | | |
|  | |  |  |
|  | 1 - 5 (of 14) nextResult Pages :  [1] [1]  [2 3] [2 3] |
| |
|
| |
 | Look
Ups |
| |