 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Special Imaging' found in 1 term [ ] and 0 definition [ ] and 0 definition [ ], (+ 19 Boolean[ ], (+ 19 Boolean[ ] results ] results
| 1 - 5 (of 20) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |  | |  | Searchterm 'Special Imaging' was also found in the following services: | | | | |
|  |  |
| Special Imaging |  |
| |
|
Special imaging primarily means advanced MRI techniques used for qualitative and quantitative measurement of biological metabolism as e.g., spectroscopy, perfusion imaging (PWI, ASL), diffusion weighted imaging ( DWI, DTI, DTT) and brain function ( BOLD, fMRI). This physiological magnetic resonance techniques offer insights into brain structure, function, and metabolism.
Spectroscopy provides functional information related to identification and quantification of e.g. brain metabolites.
MR perfusion imaging has applications in stroke, trauma, and brain neoplasm. MRI provides the high spatial and temporal resolution needed to measure blood flow to the brain. arterial spin labeling techniques utilize the intrinsic protons of blood and brain tissue, labeled by special preparation pulses, rather than exogenous tracers injected into the blood.
MR diffusion tensor imaging characterizes the ability of water to spread across the brain in different directions. Diffusion parallel to nerve fibers has been shown to be greater than diffusion in the perpendicular direction. This provides a tool to study in vivo fiber connectivity in brain MRI.
FMRI allows the detection of a functional activation in the brain because cortical activity is intimately related to local metabolism changes. See also Diffusion Tensor Tractography. | |  | | | | • Share the entry 'Special Imaging':    | | |
• View the NEWS results for 'Special Imaging' (14).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
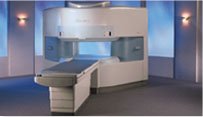
From Hitachi Medical Systems America, Inc.;
the AIRIS made its debut in 1995. Hitachi followed up with the AIRIS II system, which has proven equally successfully. 'All told, Hitachi has installed more than 1,000 MRI systems in the U.S., holding more than 17 percent of the total U.S. MRI installed base, and more than half of the installed base of open MR systems,' says Antonio Garcia, Frost and Sullivan industry research analyst.
Now Altaire employs a blend of innovative Hitachi features called VOSI™ technology, optimizing each sub-system's performance in concert with the
other sub-systems, to give the seamless mix of high-field performance
and the patient comfort, e specially for claustrophobic patients, of open MR systems.
Device Information and Specification
CLINICAL APPLICATION
Whole body
DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP)
SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 3.6 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 8 - 250msec IR: 5.2 -7,680msec GE: 1.8 - 2,000 msec FSE: 5.2 - 7,680
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
COOLING SYSTEM TYPE
Water-cooled
3.1 m lateral, 3.6 m vertical
| |  | |
• View the DATABASE results for 'Altaire™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
IMAGING MODES
Single, multislice, volume study, fast scan, multi slab
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240
| |  | |
• View the DATABASE results for 'C-SCAN™' (4).
| | | | |
|  |  | Searchterm 'Special Imaging' was also found in the following services: | | | | |
|  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
(July 20, 2009 - EPIX Pharmaceuticals, Inc. announced today that, in light of the company's lack of capital and inability to obtain additional financing or consummate a strategic transaction, it has entered into an Assignment for the Benefit of Creditors, effective immediately, in accordance with Massachusetts law).
EPIX has been a specialty pharmaceutical firm developing targeted contrast agents to improve the capability of MRI as a diagnostic tool for a variety of diseases. Gadofosveset trisodium (formerly MS-325, Vasovist™, now ABLAVARt™), is an injectable intravascular contrast agents designed for multiple vascular imaging applications, including peripheral vascular disease and coronary artery disease.
EPIX conducted a pivotal Phase III trial for the detection of peripheral vascular disease, as well as a Phase II feasibility trial for coronary artery disease diagnosis.
To ensure rapid development and adoption of gadofosveset trisodium into clinical practice upon regulatory approval, EPIX pursued an aggressive product development plan and commercialization strategy. The Company established an exclusive, worldwide sales and marketing agreement with Bayer Schering Pharma AG. EPIX also established corporate collaborations with GE Healthcare, Philips Medical Systems and Siemens Medical Systems, the three leading MRI manufacturers, which together account for approximately 80 percent of the MRI machines installed worldwide.
EPIX had other MRI contrast agents under development, most significantly a novel prototype blood clot agent ( EP-2104R). Potential clinical applications for this type of agent include detection of deep venous thrombosis, pulmonary embolism and blood clots in the coronary and carotid arteries. Currently, there is no high resolution imaging technique to directly visualize blood clots in patients with suspected cardiovascular disease.
| |  | |
• View the DATABASE results for 'EPIX Pharmaceuticals, Inc.' (7).
| | |
• View the NEWS results for 'EPIX Pharmaceuticals, Inc.' (69).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
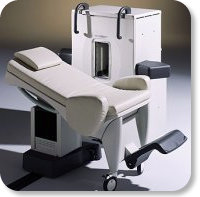
Developed by GE Lunar; the ARTOSCAN™-M is designed specifically for in-office musculoskeletal imaging. ARTOSCAN-M's compact, modular design allows placing within a clinical environment, bringing MRI to the patient. Patients remain outside the magnet at all times during the examinations, enabling constant patient-technologist contact. ARTOSCAN-M requires no special RF room, magnetic shielding, special power supply or air conditioning.
The C-SCAN™ (also known as Artoscan C) is developed from the ARTOSCAN™ - M, with a new computer platform.
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
SLICE THICKNESS
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240V
| |  | |
• View the DATABASE results for 'ARTOSCAN™ - M' (3).
| | | | |
|  | |  |  |
|  | 1 - 5 (of 20) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |
| |
|
| |
 | Look
Ups |
| |