 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | 'Toshiba America Medical Systems Inc' | |
Result : Searchterm 'Toshiba America Medical Systems Inc' found in 1 term [ ] and 7 definitions [ ] and 7 definitions [ ], (+ 1 Boolean[ ], (+ 1 Boolean[ ] results ] results
| previous 6 - 9 (of 9) Result Pages :  [1] [1]  [2] [2] |  | |  | Searchterm 'Toshiba America Medical Systems Inc' was also found in the following service: | | | | |
|  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | | | |
|  | |  |  |  |
| |
|
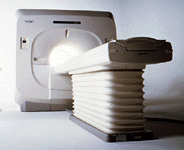
From Toshiba America Medical Systems Inc.;
VISART™ series is a 1.5 Tesla superconducting MRI system that has been designed to meet the expanding role of MRI in today's clinical environment. The system utilizes innovative technologies such as digital RF, high speed actively shielded gradients and optimized RF coils, which support a wide range of MRI developments. The Visart, an early type of Toshiba Medical Systems Inc., can be retrofitted or upgraded to the Excelart configuration.
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Closed-loop water-cooled | |  | |
• View the DATABASE results for 'VISART™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
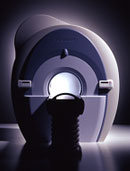
From Toshiba America Medical Systems Inc.;
With its high-field strength, the Vantage™ delivers the clinical capabilities and image quality expected by cardiologists, while simultaneously offering patients a more comfortable and non-invasive option, said Dane Peshe, director, MRI Business Unit, Toshiba America Medical Systems. Vantage™ supports a full complement of cardiovascular imaging studies, ranging from stroke evaluation to peripheral vascular imaging. Additionally, the ultra short bore design offers patients a greater feeling of openness that reduces claustrophobic sensations, while Toshiba's exclusive, patented Pianissimo™ technology reduces scan noise by as much as 90 percent for a more pleasant experience.'
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Ultra short bore SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, EPI, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study 32-1024, phase;; 64-1024, freq. POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Closed-loop water-cooled Liquid helium: approx. less than 0.05 L/hr Passive, active, auto-active | |  | |
• View the DATABASE results for 'Vantage™' (2).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | Searchterm 'Toshiba America Medical Systems Inc' was also found in the following service: | | | | |
|  |  |
| |
|
Silicon Graphics workstations are used in products from many other industry-leading medical manufacturers including:
The CT and MR products from General Electric Medical Systems uses Silicon Graphics® O2 and OCTANE workstation.
From Siemens Medical Systems, Inc., the postprocessing CT and MR workstations use Silicon Graphics O2 workstations.
Toshiba America MRI Inc. uses Silicon Graphics O2 workstations in its MRI scanners.
Bruker uses Silicon Graphics O2 workstations in its MRI scanners. | |  | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |