 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Ultra' was also found in the following services: | | | | |
|  |  |
| |
|
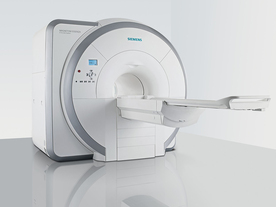
From Siemens Medical Systems;
Received FDA clearance in 2007.
The MAGNETOM Essenza is designed to combine high system performance with simple installation and power requirements to provide optimal operating costs for limited budgets. The standard system has up to 25 integrated coil elements and 8 independent radio frequency channels. Tim allows the combination of up to 4 different coils that reduce patient and coil repositioning.
The 1.5 Tesla system is designated for a complete range of clinical applications, including neurology, orthopedics, body imaging, angiography, cardiology, breast imaging, oncology and pediatric MRI.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, TMJ optional.
CHANNELS (min. / max. configuration)
8, 16
MAGNET WEIGHT (gantry included)
4350 kg in operation
DIMENSION H*W*D (gantry included)
145 x 226 x 216 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
30 mT/m, 300 msec to 10 mT/m
Passive, active; first order standard
second order optional
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 45 kVA
| |  | | | |
|  | |  |  |  |
| |
|
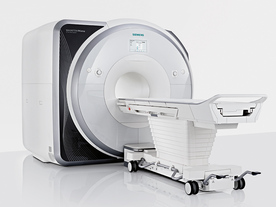
From Siemens Medical Systems;
Received FDA clearance in 2013.
The MAGNETOM Prisma is the 3T PowerPack for exploration that offers most demanding clinical and research challenges of today and the future. The latest parallel transmit technology, TimTX TrueShape, enables zooming into specific body regions for enhanced image quality. Furthermore, the Tim 4G integrated coil technology offers remarkable imaging flexibility and supports complex
examinations across the whole body.
Onsite upgrades to the MAGNETOM Prisma for customers who have already installed the 3 Tesla MAGNETOM Trio are possible.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
64, 128
MAGNET WEIGHT (gantry included)
13000 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground;
| |  | | | |
|  | |  |  |  |
| |
|
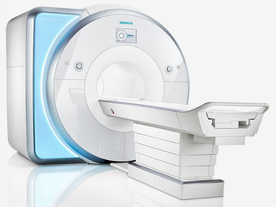
From Siemens Medical Systems;
Received FDA clearance in 2010.
MAGNETOM Skyra is a top-of-the-line, patient friendly wide bore 3 Tesla MRI system.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix and Day optimizing throughput), to enhance both productivity and image quality with the complete range of advanced applications for clinical routine and research. Tim 4G features lighter, trimmer MRI coils that take up less space inside the magnet but deliver a high coil element density with increased signal to noise ratio and the possibility to use high iPAT factors.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
48, 64, 128
Chemical shift imaging, single voxel spectroscopy
MINIMUM TE
3D T1 spoiled GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
5768 kg
DIMENSION H*W*D (gantry included)
173 x 231 x 219 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 110 kVA
| |  | | | |
|  |  | Searchterm 'Ultra' was also found in the following services: | | | | |
|  |  |
| |
|
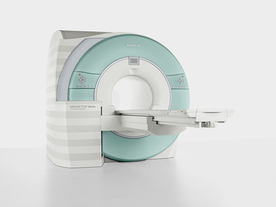
From Siemens Medical Systems;
Received FDA clearance in 2007.
The MAGNETOM Verio provides up to 102 integrated matrix coil elements and up to 32 independent radiofrequency channels that allow flexible coil combinations to make patient and coil repositioning virtually unnecessary. The Tim (total imaging matrix) technology also increases patient throughput due to a shorter scan time.
The open bore design offers great comfort for patients of all shapes and sizes.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short open bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, TMJ optional.
CHANNELS (min. / max. configuration)
8, 18, 32
Chemical shift imaging, single voxel spectroscopy
MAGNET WEIGHT (gantry included)
8200 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order standard
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 110 kVA
| |  | | | |
|  | |  |  |  |
| |
|
| |  | |
• View the DATABASE results for 'MR Guided Interventions' (8).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
|  |
AI analysis finds younger AFib patients benefit from MRI-guided ablation treatments
Friday, 25 August 2023 by www.eurekalert.org |  |  |
Theranostic nano-platform for MRI-guided synergistic therapy against breast cancer
Monday, 26 September 2022 by phys.org |  |  |
Magnetic seeds used to heat and kill cancer
Tuesday, 1 February 2022 by www.sciencedaily.com |  |  |
What is the effect of MRI with targeted biopsies on the rate of patients discontinuing active surveillance? A reflection of the use of MRI in the PRIAS study
Thursday, 8 April 2021 by www.docwirenews.com |  |  |
Modeling of Active Shimming of Metallic Needles for Interventional MRI
Monday, 29 June 2020 by pubmed.ncbi.nlm.nih.gov |  |  |
Magnetic Resonance Imaging Guided Confirmatory Biopsy for Initiating Active Surveillance of Prostate Cancer
Wednesday, 11 September 2019 by jamanetwork.com |  |  |
FDA clears ViewRay's next-gen, MRI-guided radiation therapy device
Tuesday, 28 February 2017 by www.fiercebiotech.com |  |  |
Siemens, U. of Twente Biopsy Robot Promises Greater Precision, Less Cost
Friday, 22 January 2016 by www.meddeviceonline.com |  |  |
Magnetic resonance-guided motorized transcranial ultrasound system for blood-brain barrier permeabilization along arbitrary trajectories in rodents
Thursday, 24 December 2015 by www.ncbi.nlm.nih.gov |  |  |
New MRI-Guided Catheter Shows Major Potential for Stroke Treatment
Tuesday, 29 December 2015 by www.radiology.ucsf.edu |  |  |
Polish study on MRI-ultrasound for targeted prostate biopsy wins CEM award
Tuesday, 12 November 2013 by medicalxpress.com |  |  |
C4 Imaging Announces FDA 510(k) Clearance of its Positive-Signal MRI Marker - Sirius™
Friday, 6 December 2013 by www.digitaljournal.com |
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |