 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Ultra' was also found in the following services: | | | | |
|  |  |
| |
|
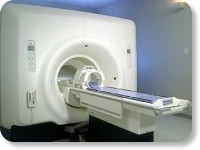
(Signa VH/i 3.0T)
With GE Healthcare
leading-edge technology in ultra-high-field imaging. The 3 T VH/i provides a platform for advanced applications in radiology, cardiology, psychology and psychiatry. Real-time image processing lets you acquire multislice whole brain images and map brain functions for research or surgical planning. And the 3 T Signa VH/i is flexible enough to provide clinicians with high performance they require. It can provide not only outstanding features in brain scanning and neuro-system research, but also a wide range of use in scanning breasts, extremities, the spine and the cardiovascular systems.
Device Information and Specification CLINICAL APPLICATION Whole body
T/R quadrature head, T/R quadrature body, T/R phased array extremity (opt) SE, IR, 2D/3D GRE, FGRE, RF-spoiled GRE, FSE, Angiography: 2D/3D TOF, 2D/3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer 100 Images/sec with Reflex100 MULTISLICE 100 Images/sec with Reflex100 2D 0.5-100mm in 0.1mm incremental 128x512 steps 32 phase encode H*W*D 260cm x 238cm x 265cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. Less than 0.14 L/hr liquid He | |  | | | |
|  | |  |  |  |
| |
|
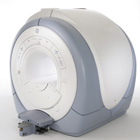
From GE Healthcare;
The Signa HDx MRI system is GE's leading edge whole body magnetic resonance scanner designed to support high resolution, high signal to noise ratio, and short scan times.
Signa HDx 3.0T offers new technologies like ultra-fast image reconstruction through the new XVRE recon engine, advancements in parallel imaging algorithms and the broadest range of premium applications. The HD applications, PROPELLER (high-quality brain imaging extremely resistant to motion artifacts), TRICKS (contrast-enhanced angiographic vascular lower leg imaging), VIBRANT (for breast MRI), LAVA (high resolution liver imaging with shorter breath holds and better organ coverage) and MR Echo (high-definition cardiac images in real time) offer unique capabilities.
Device Information and Specification CLINICAL APPLICATION Whole body
CONFIGURATION Compact short bore SE, IR, 2D/3D GRE, RF-spoiled GRE, 2DFGRE, 2DFSPGR, 3DFGRE, 3DFSPGR, 3DTOFGRE, 3DFSPGR, 2DFSE, 2DFSE-XL, 2DFSE-IR, T1-FLAIR, SSFSE, EPI, DW-EPI, BRAVO, Angiography: 2D/3D TOF, 2D/3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer H*W*D 240 x 2216,6 x 201,6 cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. | |  | | | |
|  | |  |  |  |
| |
|
Sinerem® is the brand name (same as Combidex®) for an ultrasmall superparamagnetic iron oxide ( USPIO) to detect metastatic disease in lymph nodes. Metastatic nodes show less uptake of this MRI contrast agent, which results in less signal decrease and allows the differentiation of normal lymph nodes from normal-sized, metastatic nodes.
Lymph node imaging with Sinerem® is performed 24 to 36 hours after slow infusion. Normal lymph nodes turn black post contrast, namely on T2* weighted images. Metastatic lymph nodes remain unchanged in signal intensity.
Indication and Diseases: Cancer, Imaging for diagnosis, Lymphatic disorders.
See Ferumoxtran, and Classifications, Characteristics, etc.
Guerbet decided in 2007 to withdraw its Marketing Authorisation Application
(MAA) for Sinerem.Drug Information and Specification r1=25, r2=160, B0=0.47T, r1=23.3, r2=48.9, B0=0.47T PHARMACOKINETIC Vascular, lymph v. hepatocyte (AG-USPIO) PREPARATION Suspend in an isotonic glucose solution DO NOT RELY ON THE INFORMATION PROVIDED HERE, THEY ARE
NOT A SUBSTITUTE FOR THE ACCOMPANYING PACKAGE INSERT! | |  | |
• View the DATABASE results for 'Sinerem®' (6).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Ultra' was also found in the following services: | | | | |
|  |  |
| |
|
(SAR) The Specific Absorption Rate is defined as the RF power absorbed per unit of mass of an object, and is measured in watts per kilogram (W/kg).
The SAR describes the potential for heating of the patient's tissue due to the application of the RF energy necessary to produce the MR signal. Inhomogeneity of the RF field leads to a local exposure where most of the absorbed energy is applied to one body region rather than the entire person, leading to the concept of a local SAR. Hot spots may occur in the exposed tissue, to avoid or at least minimize effects of such theoretical complications, the frequency and the power of the radio frequency irradiation should be kept at the lowest possible level. Averaging over the whole body leads to the global SAR.
It increases with field strength, radio frequency power and duty cycle, transmitter-coil type and body size. The doubling of the field strength from 1.5 Tesla (1.5T) to 3 Tesla ( 3T) leads to a quadrupling of SAR. In high and ultrahigh fields, some of the multiple echo, multiple-slice pulse sequences may create a higher SAR than recommended by the agencies. SAR can be reduced by lower flip angle and longer repetition times, which could potentially affect image contrast.
Normally no threatening increase in temperature could be shown. Even in high magnetic fields, the local temperature increases not more than 1°C. 2.1°C is the highest measured increase in skin temperature. Eddy currents may heat up implants and thus may cause local heating.
FDA SAR limits:
•
Whole body: 4W/kg/15-minute exposure averaged;
•
Head: 3W/kg/10-minute exposure averaged;
•
Head or torso: 8W/kg/5 minute exposure per gram of tissue;
•
Extremities: 12W/kg/5 minute exposure per gram of tissue.
IEC (International Electrotechnical Commission) SAR limits of some European countries:
All limits are averaged over 6 minutes.
•
Level 0 (normal operating mode): Whole body 2W/kg; Head 3.2W/kg; Head or Torso (local) 10W/kg;
Extremities (local) 20W/kg;
•
Level I (first level controlled operating mode): Whole body 4W/kg; Head 3.2W/kg; Head or Torso (local) 10W/kg; Extremities (local) 20W/kg;
•
Level II (second level controlled operating mode): All values are over Level I values.
(For more details: IEC 60601-2-33 (2002))
In most countries standard MRI systems are limited to a maximum SAR of 4 W/kg, so most scanning in level II is impossible.
For Level I, in addition to routine monitoring, particular caution must be exercised for patients who are sensitive to temperature increases or to RF energy.
For Japan different SAR limits are valid. | |  | |
• View the DATABASE results for 'Specific Absorption Rate' (8).
| | |
• View the NEWS results for 'Specific Absorption Rate' (1).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
Tumor specific MRI contrast agents are in development to provide better delineation and progression information for various tumors. Clinical oncology has a need for contrast agents that can identify tumors and metastases at a size of 100,000 cells rather than 1,000,000,000 cells. This level of sensitivity requires excellent tumor targeting of imaging agents and a high MRI signal.
Tumor specific agents accumulate at pathological tissues by passive or active targeting mechanisms. Passive targeting agents use e.g., the natural defense mechanisms in which phagocytic cells remove foreign particles from the body. Active targeting is based on a ligand-directed, site-specific accumulation of contrast agents. The availability of macromolecular contrast agents such as feruglose and ultrasmall superparamagnetic iron oxide ( USPIO), which permit the assessment of tissue permeability, may also improve the detection of tumor grade, tumor type, and response to drugs that target angiogenesis.
See also Monoclonal Antibodies, Metalloporphyrins, Nitroxides and Ferrioxamine. | |  | |
• View the DATABASE results for 'Tumor Specific Agents' (6).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |