 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'hitachi' found in 1 term [ ] and 4 definitions [ ] and 4 definitions [ ] ]
| 1 - 5 (of 5) Result Pages :  [1] [1] |  | |  | Searchterm 'hitachi' was also found in the following services: | | | | |
|  |  |
| |
|
 [This entry is marked for removal.]
Hitachi
[This entry is marked for removal.]
Hitachi Medical Systems America, Inc. (HMSA), was a major provider of magnetic resonance imaging systems in the United States. Hitachi had more than 2300 installed permanent magnet MR imaging systems worldwide. As a full-line supplier of medical imaging equipment in Japan, Hitachi Medical Corporation (HMC) founded HMSA to provide a direct link to the U.S. marketplace. Altaire™ , the open MR system from Hitachi, extends the family of open MRI products.
In December 2019 Japan's Fujifilm announced the acquisition of Hitachi's diagnostic imaging business for 179 billion yen ($1.63 billion). This includes Hitachi’s CT, MRI, X-ray, and ultrasound imaging operations, also its electronic health record business. Fujifilm expects the deal to close in July 2020 subject to regulatory clearances.
MRI Scanners:
| |  | | | | • Share the entry 'Hitachi Medical Systems America Inc.(USA)/Hitachi Medical Corp.(Tokyo)':    | | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
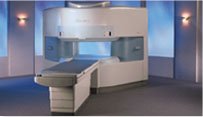
From Hitachi Medical Systems America, Inc.;
the AIRIS made its debut in 1995. Hitachi followed up with the AIRIS II system, which has proven equally successfully. 'All told, Hitachi has installed more than 1,000 MRI systems in the U.S., holding more than 17 percent of the total U.S. MRI installed base, and more than half of the installed base of open MR systems,' says Antonio Garcia, Frost and Sullivan industry research analyst.
Now Altaire employs a blend of innovative Hitachi features called VOSI™ technology, optimizing each sub-system's performance in concert with the
other sub-systems, to give the seamless mix of high-field performance
and the patient comfort, especially for claustrophobic patients, of open MR systems.
Device Information and Specification
CLINICAL APPLICATION
Whole body
DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP)
SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 3.6 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 8 - 250msec IR: 5.2 -7,680msec GE: 1.8 - 2,000 msec FSE: 5.2 - 7,680
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
COOLING SYSTEM TYPE
Water-cooled
3.1 m lateral, 3.6 m vertical
| |  | |
• View the DATABASE results for 'Altaire™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|

From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0.3 T field-strength magnet and phased array coils deliver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  |  | Searchterm 'hitachi' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
SE, IR, FSE, FIR, GE, SG, BASG, PBSG, PCIR, DWI, Radial, Angiography: TOF, FLUTE (Fluoro-triggered bolus MRA), Time-resolved MRA
IMAGING MODES
Single, multislice, volume study
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
| |  | |
• View the DATABASE results for 'Echelon™ 1.5T' (2).
| | |
• View the NEWS results for 'Echelon™ 1.5T' (3).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
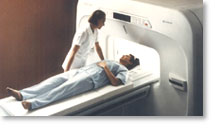
From Hitachi Medical Systems America, Inc.; because of its dependability, the MRP-7000™ remains popular more than a decade after the first U.S. system was shipped. This system maintains a high resale value, what has made it one of the most sought-after scanners on the used MRI equipment market.
Device Information and Specification CLINICAL APPLICATION Whole body DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP) SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOFIMAGING MODES Single, multislice, volume study horizontal 2.5 m x 2.1 m vertical | |  | |
• View the DATABASE results for 'MRP-7000™' (2).
| | | | |
|  | |  |  |
|  | |
|  | 1 - 5 (of 5) Result Pages :  [1] [1] |
| |
|
| |
 | Look
Ups |
| |