 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'sense' found in 2 terms [ ] and 25 definitions [ ] and 25 definitions [ ] ]
| previous 16 - 20 (of 27) nextResult Pages :  [1] [1]  [2 3 4 5 6] [2 3 4 5 6] |  | |  | Searchterm 'sense' was also found in the following services: | | | | |
|  |  |
| |
|
| | | |  | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
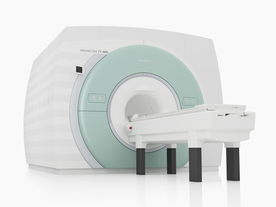
From Siemens Medical Systems;
The MAGNETOM 7T is designed as an open research platform. 7T MRI provides anatomical detail at the submillimiter scale, enhanced contrast mechanisms, outstanding spectroscopy performance, ultra-high resolution functional imaging ( fMRI), multinuclear whole-body MRI and functional information.
This ultra high field (UHF) MRI device is a research system and not cleared, approved or licensed in any jurisdiction for patient examinations.
Device Information and Specification
CLINICAL APPLICATION
Whole body
High-performance, ultra high field coils available. Integration and support for coil developments.
CHANNELS (min. / max. configuration)
32, optional 8 channels TX array
40 x 40 x 30 cm³ less than 8% nonlinearity
MAGNET WEIGHT (gantry included)
35017 kg
DIMENSION H*W*D (gantry included)
320 x 240 x 317,5 cm
MAX. AMPLITUDE
up to 70 mT/m
Up to 3rd order shim coils, user configurable B0 shim ? B0 maps and ROI definition
POWER REQUIREMENTS
2000 Volts, 650A
| |  | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
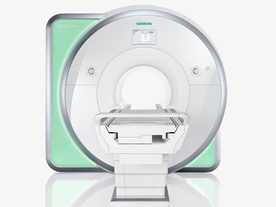
From Siemens Medical Systems;
Received FDA clearance in 2010.
The MAGNETOM Aera is a patient friendly, comfortable 1.5 Tesla MRI system with advanced radio frequency chain.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix + Day optimizing throughput), to enhance both productivity and image quality.
Tim 4G technology provides improved SNR. The standard system configuration of 48 radio frequency channels and 204 coil elements creates an imaging matrix that allows maximum use of coil elements at full field of view. Dot provides improved image consistency through new features like auto align, auto FoV and automatic bolus detection.
Device Information and Specification
CLINICAL APPLICATION
Whole body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder. Up to 60% more SNR with Tim 4G.
CHANNELS (min. / max. configuration)
48, 64
MINIMUM TE
3-D GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
3121 kg
DIMENSION H*W*D (gantry included)
145 x 231 x 219 cm
MAX. AMPLITUDE
33 or 45 mT/m
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 85 kVA
| |  | | | |
|  |  | Searchterm 'sense' was also found in the following services: | | | | |
|  |  |
| |
|
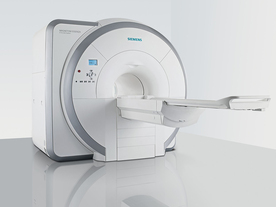
From Siemens Medical Systems;
Received FDA clearance in 2007.
The MAGNETOM Essenza is designed to combine high system performance with simple installation and power requirements to provide optimal operating costs for limited budgets. The standard system has up to 25 integrated coil elements and 8 independent radio frequency channels. Tim allows the combination of up to 4 different coils that reduce patient and coil repositioning.
The 1.5 Tesla system is designated for a complete range of clinical applications, including neurology, orthopedics, body imaging, angiography, cardiology, breast imaging, oncology and pediatric MRI.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, TMJ optional.
CHANNELS (min. / max. configuration)
8, 16
MAGNET WEIGHT (gantry included)
4350 kg in operation
DIMENSION H*W*D (gantry included)
145 x 226 x 216 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
30 mT/m, 300 msec to 10 mT/m
Passive, active; first order standard
second order optional
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 45 kVA
| |  | | | |
|  | |  |  |  |
| |
|
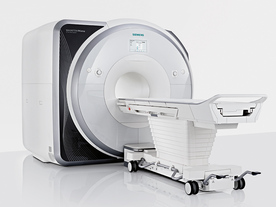
From Siemens Medical Systems;
Received FDA clearance in 2013.
The MAGNETOM Prisma is the 3T PowerPack for exploration that offers most demanding clinical and research challenges of today and the future. The latest parallel transmit technology, TimTX TrueShape, enables zooming into specific body regions for enhanced image quality. Furthermore, the Tim 4G integrated coil technology offers remarkable imaging flexibility and supports complex
examinations across the whole body.
Onsite upgrades to the MAGNETOM Prisma for customers who have already installed the 3 Tesla MAGNETOM Trio are possible.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
64, 128
MAGNET WEIGHT (gantry included)
13000 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground;
| |  | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |