 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'stir' found in 0 term [ ] and 49 definitions [ ] and 49 definitions [ ] ]
| previous 16 - 20 (of 49) nextResult Pages :  [1 2 3 4 5 6 7 8 9 10] [1 2 3 4 5 6 7 8 9 10] |  | |  | Searchterm 'stir' was also found in the following services: | | | | |
|  |  |
| |
|
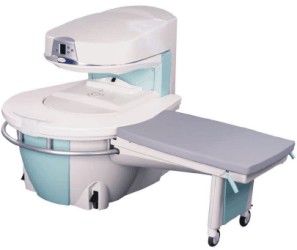
Manufactured by Esaote S.p.A.;
a low field open MRI scanner with permanent magnet for orthopedic use. The outstanding feature of this MRI system is a patient friendly design with 24 cm diameter, which allows the imaging of extremities and small body parts like shoulder MRI. The power consumption is around 1.3 kW and the needed minimum floor space is an area of 16 sq m.
At RSNA 2006 Hologic Inc. introduced a new dedicated extremity MRI scanner, the Opera. Manufactured by Esaote is the Opera a redesign of Esaote's 0.2 Tesla E-Scan XQ platform, which now enables complete imaging of all extremities, including hip and shoulder applications. 'Real-time positioning' reportedly speeds patient setup and reduces exam times.
Esaote North America and Hologic Inc are the U.S. distributors of this MRI device.
Device Information and Specification CLINICAL APPLICATION Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE- STIR, 3D GE, ME, TME, HSE IMAGING MODES Single, multislice, volume study, fast scan, multi slab2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm 4096 gray lvls, 256 lvls in 3D POWER REQUIREMENTS 2,0 kW; 110/220 V single phase | |  | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|

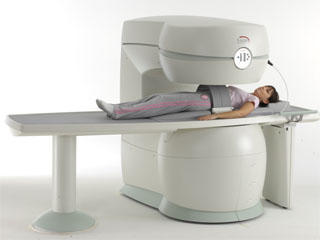
From Philips Medical Systems;
the Panorama 0.23 T, providing a new design optimized for patient comfort, faster reconstruction time than before (300 images/second) and new gradient
specifications. Philips' Panorama 0.23 T I/T supports MR-guided interventions, resulting in minimally invasive procedures, more targeted surgery, reduced recovery time and shorter hospital stays. Optional OptoGuide functionality enables real-time needle tracking. Philips' Panorama 0.23 TPanorama 0.2 R/T is the first and only open MRI system to enable radiation therapy planning using MR data sets. The Panorama also features the new and consistent Philips User Interface, an essential element of the Vequion clinical IT family of products and services.
Device Information and Specification CLINICAL APPLICATION Whole body SE, FE, IR, FFE, DEFFE, DESE, TSE, DETSE, Single shot SE, DRIVE, Balanced FFE, MRCP, Fluid Attenuated Inversion Recovery, Turbo FLAIR, IR-TSE, T1- STIR TSE, T2- STIR TSE, Diffusion Imaging, 3D SE, 3D FFE, MTC;; Angiography: CE-ANGIO, MRA 2D, 3D TOFOpen x 46 cm x infinite (side-first patient entry) POWER REQUIREMENTS 400/480 V COOLING SYSTEM TYPE Closed loop chilled water ( chiller included) | |  | |
• View the DATABASE results for 'Panorama 0.23T™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
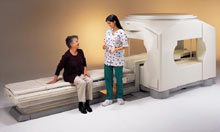
From Esaote S.p.A.;
Esaote introduced the S-SCAN at RSNA in November 2007. The S-SCAN is a dedicated joint and spine MR scanner derived from the company's earlier G-SCAN system. Unlike the G-SCAN, neither the patient table nor
the magnet can rotate from horizontal to vertical position. The patient table can only moved manually. Improved electronics, new coils for lumbar and cervical spine, new pulse sequences, a modified version of the magnet poles and gradient coils are used with a new software release in the S-SCAN.
Esaote North America is the exclusive U.S. distributor of this MRI device.
Device Information and Specification SE, GE, IR, STIR, TSE, 3D CE, GE- STIR, 3D GE, ME, TME, HSE POWER REQUIREMENTS 3 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'S-SCAN' (3).
| | |
• View the NEWS results for 'S-SCAN' (1).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'stir' was also found in the following services: | | | | |
|  |  |
| |
|
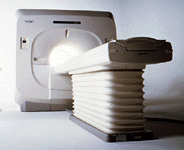
From Toshiba America Medical Systems Inc.;
the Ultra™ system was developed to help healthcare providers be more competitive by delivering greater patient comfort and a broad range of clinical capabilities, says Anita Bowler, product manager, MRI Business Unit, Toshiba America Medical Systems Inc With its unique, powerful gradient technology, the Ultra™ performs advanced clinical studies and consistently provides high-resolution images that are typically associated with high field MRI systems. At the same time, the Ultra™ offers a truly open feeling that makes patients more relaxed, especially those with claustrophobic tendencies.
Device Information and Specification CLINICAL APPLICATION Whole Body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI, Single shot EPI diffusion, True SSFP, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC, Black Blood MRA POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | | | |
|  | |  |  |  |
| |
|
From Toshiba America Medical Systems Inc.;
VISART™ series is a 1.5 Tesla superconducting MRI system that has been designed to meet the expanding role of MRI in today's clinical environment. The system utilizes innovative technologies such as digital RF, high speed actively shielded gradients and optimized RF coils, which support a wide range of MRI developments. The Visart, an early type of Toshiba Medical Systems Inc., can be retrofitted or upgraded to the Excelart configuration.
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Closed-loop water-cooled | |  | |
• View the DATABASE results for 'VISART™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |