 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | 'toshiba america medical systems inc' | |
Result : Searchterm 'toshiba america medical systems inc' found in 1 term [ ] and 7 definitions [ ] and 7 definitions [ ], (+ 1 Boolean[ ], (+ 1 Boolean[ ] results ] results
| 1 - 5 (of 9) nextResult Pages :  [1] [1]  [2] [2] |  | |  | Searchterm 'toshiba america medical systems inc' was also found in the following service: | | | | |
|  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
Toshiba Medical Systems started 1914 and was a leading diagnostic imaging manufacturer with departments including research, development, production, service and support of medical imaging equipment and systems. 1976 Toshiba America Medical Systems, Inc. (TAMS) was founded to coordinate sales and service for previously established business operations in the USA.
In March 2016 Toshiba sold its Toshiba Medical Systems for $6 billion to Japan's Canon. After regulatory approval the company got renamed to Canon Medical Systems.
MRI Scanners:
0.2T to 1.0T:
1.5T:
3.0T:
| |  | | | | • Share the entry 'Toshiba America Medical Systems Inc.':    | | |
• View the NEWS results for 'Toshiba America Medical Systems Inc.' (10).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
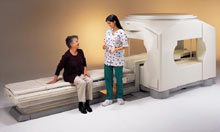
From Toshiba America Medical Systems Inc.;
the Ultra™ system was developed to help healthcare providers be more competitive by delivering greater patient comfort and a broad range of clinical capabilities, says Anita Bowler, product manager, MRI Business Unit, Toshiba America Medical Systems Inc With its unique, powerful gradient technology, the Ultra™ performs advanced clinical studies and consistently provides high-resolution images that are typically associated with high field MRI systems. At the same time, the Ultra™ offers a truly open feeling that makes patients more relaxed, especially those with claustrophobic tendencies.
Device Information and Specification CLINICAL APPLICATION Whole Body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI, Single shot EPI diffusion, True SSFP, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC, Black Blood MRA POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | | | |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
Opt. (WIP) Single and Multi Voxel
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 1.2 msec min. FE
less than 0.015 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
4050 kg (bare magnet incl. L-He)
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart AG™ with Pianissimo' (2).
| | | | |
|  |  | Searchterm 'toshiba america medical systems inc' was also found in the following service: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 0.9 msec min. FE
less than 0.011 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
4050 kg (bare magnet incl. L-He)
POWER REQUIREMENTS
380/400/415/440/480 V
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart XG™ with Pianissimo' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
From Toshiba America Medical Systems Inc.;
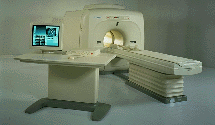
FLEXART™ series is a 0.5 T superconducting MRI system that has been designed to meet the expanding role of MRI in today's clinical environment. The system utilizes innovative technologies such as digital RF, high speed actively shielded gradients and optimized RF coils which support a wide range of MRI developments.
Device Information and Specification
CLINICAL APPLICATION
Whole body
Quadrature, solenoid and multi-channel configurations
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
POWER REQUIREMENTS
380/400/415/440/480 V
COOLING SYSTEM TYPE
Closed-loop water-cooled
| |  | |
• View the DATABASE results for 'FLEXART™' (2).
| | | | |
|  | |  |  |
|  | |
|  | 1 - 5 (of 9) nextResult Pages :  [1] [1]  [2] [2] |
| |
|
| |
 | Look
Ups |
| |