 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  |
|  |  |
 |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | |
• View the DATABASE results for 'OPART™' (2).
| | | | | |  |
 |
| |
| Open MRI scanners have been developed for people who are anxious or obese or for examination of small parts of the body, such as the extremities ( knee, shoulder). In addition, some systems offer imaging in different positions and sequences of movements.
The basic technology of an open MRI machine is similar to that of a traditional MRI device.
The major difference for the patient is that instead of lying in a narrow tunnel, the imaging table has more space around the body so that the magnet does not completely surround the person being tested.
Types of constructions:
•
Semi open high field MRI scanners provide an ultra short bore (tunnel) and widely flared ends. In this type of MRI systems, patients lie with the head in the space outside the bore, if for example the hips are examined.
•
Open low field MRI machines have often a wide open design, e.g. an open C-arm scanner is shaped like two large discs separated by a large pillar. Patients have an open sided feeling and more space around them allows a wider range of positions.
•
Advanced open MRI scanners combine the advantages of both, the high field strength, newest gradient technology and wide open design. Even scans of patients in upright, weight-bearing positions are possible (e.g. Upright™ MRI formerly Stand-Up MRI).
Difficulties with a traditional MRI scan include claustrophobia and patient size or, for health related reasons, patients who are not able to receive this type of diagnostic test. The MRI unit is a limited space, and some patients may be too large to fit in a narrow tunnel. In addition, weight limits can restrict the use of some scanners. The open MRI magnet has become the best option for those patients.
All of the highest resolution MRI scanners are tunnels and tend to accentuate the claustrophobic reaction. While patients may find the open MRI scanners easier to tolerate, some machines use a lower field magnet and generates lower image quality or have longer scan time. The better performance of an advanced open MRI scanner allows good image quality caused by the higher signal to noise ratio with maximum patient comfort.
See also Claustrophobia, MRI scan and Knee MRI. | |  | |
• View the NEWS results for 'Open MRI' (16).
| | |
• View the DATABASE results for 'Open MRI' (37).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| | |  | |  |  |
 |
| |
|
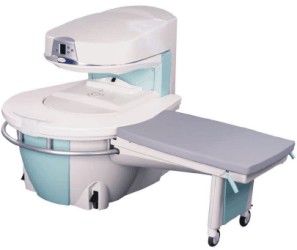
Manufactured by Esaote S.p.A.;
a low field open MRI scanner with permanent magnet for orthopedic use. The outstanding feature of this MRI system is a patient friendly design with 24 cm diameter, which allows the imaging of extremities and small body parts like shoulder MRI. The power consumption is around 1.3 kW and the needed minimum floor space is an area of 16 sq m.
At RSNA 2006 Hologic Inc. introduced a new dedicated extremity MRI scanner, the Opera. Manufactured by Esaote is the Opera a redesign of Esaote's 0.2 Tesla E-Scan XQ platform, which now enables complete imaging of all extremities, including hip and shoulder applications. 'Real-time positioning' reportedly speeds patient setup and reduces exam times.
Esaote North America and Hologic Inc are the U.S. distributors of this MRI device.
Device Information and Specification CLINICAL APPLICATION Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE IMAGING MODES Single, multislice, volume study, fast scan, multi slab2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm 4096 gray lvls, 256 lvls in 3D POWER REQUIREMENTS 2,0 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'Opera (E-SCAN™ XQ)' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| | |  |
 |
| |
| An image in which the signal from two spectral components (such as fat and water) is 180° out of phase and leads to destructive interference in a voxel.
Since fat precesses slower than water, based on their chemical shift, their signals will decay and precess in the transverse plane at different frequencies. When the phase of the TE becomes opposed (180°), their combined signal intensities subtract with each other in the same voxel, producing a signal void or dark band at the fat/water interface of the tissues being examined.
Opposed phase gradient echo imaging for the abdomen is a lipid-type tissue sensitive sequence particularly for the liver and adrenal glands, which puts a signal intensity around abnormal water-based tissues or lesions that are fatty.
Due to the increased sensitivity of opposed phase, the tissue visualization increases the lesion-to-liver contrast and exhibits more signal intensity loss in tissues containing small amounts of lipids compared to a spin echo T1 with fat suppression.
Using an opposed phase gradient echo also provides the ability to differentiate various pathologies in the brain, including lipids, methaemoglobin, protein, calcifications and melanin.
See also Out of Phase, and Dixon. | | | | |  | |
• View the DATABASE results for 'Opposed Phase Image' (5).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| | |  |
 |
| |
| OptiMARK® is a formulation of a nonionic gadolinium chelate of diethylenetriamine pentaacetic acid bismethoxyethylamide ( gadoversetamide), for use as a paramagnetic MRI contrast agent.
OptiMARK® ( gadoversetamide injection) is to be administered by intravenous injection.
OptiMARK® is indicated for MRI diagnostic procedures to provide increased enhancement and visualization of lesions of the brain, spine and liver, including tumors. WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate less than 30 mL/min/1.73m 2), or acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period. Drug Information and Specification T1, Predominantly positive enhancement PHARMACOKINETIC Intravascular, extracellular DOSAGE 0.1 mmol/kg / 0.2 mL/kg PREPARATION Finished product INDICATION Neuro/whole body PRESENTATION Vials of 5, 10, 15, 20, 50 mL and
Pre-filled syringes of 10, 15, 20 and 30 mL DO NOT RELY ON THE INFORMATION PROVIDED HERE, THEY ARE
NOT A SUBSTITUTE FOR THE ACCOMPANYING PACKAGE INSERT! Distribution Information TERRITORY TRADE NAME DEVELOPMENT
STAGE DISTRIBUTOR Australia OptiMARK® for sale | |  | |
• View the DATABASE results for 'OptiMARK®' (5).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| | |  | |  |  |
| | |
|
| |
 | Look
Ups |
| |