 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm '3 Tesla' found in 0 term [ ] and 11 definitions [ ] and 11 definitions [ ], (+ 11 Boolean[ ], (+ 11 Boolean[ ] results ] results
| previous 6 - 10 (of 22) nextResult Pages :  [1 2 3] [1 2 3]  [4 5] [4 5] |  | |  | Searchterm '3 Tesla' was also found in the following services: | | | | |
|  |  |
| |
|
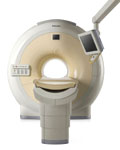
From Philips Medical Systems;
Philips continues to expand the frontiers of utra high field MRI with the introduction of the new Intera Achieva 3.0T™. Its powerful future-safe platform shares all the advantages of the Achieva family and covers applications throughout the whole body.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
Passive and dynamic, 1st order std./2nd opt.
| |  | | | | | | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body SE, IR, 2D/3D TurboSE, Turbo IR, Dark-Fluid IR, True IR, 2D/3D MEDIC, 2D/3D GRE FLASH, 2D/3D GRE FISP, 2D/3D PSIF, 2D TurboFLASH, 3D MP-RAGE, 3D TurboFLASH, 2D/3D TOF angiography, MTC, TONE with 3D TOF MRA, GMR, LOTA IMAGING MODES Single, multislice, volume study, multi angle, multi oblique178 images/sec at 256 x 256 at 100% FOV1024 x 1024 full screen display POWER REQUIREMENTS 380/400/420/440/480 V Passive, act.; 1st order std./2nd opt. | |  | | | |
|  | |  |  |  |
| |
|
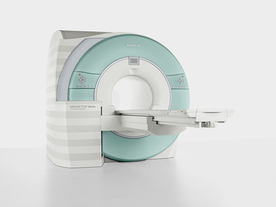
From Siemens Medical Systems;
Received FDA clearance in 2007.
The MAGNETOM Verio provides up to 102 integrated matrix coil elements and up to 32 independent radiofrequency channels that allow flexible coil combinations to make patient and coil repositioning virtually unnecessary. The Tim (total imaging matrix) technology also increases patient throughput due to a shorter scan time.
The open bore design offers great comfort for patients of all shapes and sizes.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short open bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, TMJ optional.
CHANNELS (min. / max. configuration)
8, 18, 32
Chemical shift imaging, single voxel spectroscopy
MAGNET WEIGHT (gantry included)
8200 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order standard
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 110 kVA
| |  | | | |
|  |  | Searchterm '3 Tesla' was also found in the following services: | | | | |
|  |  |
| |
|
The subacute risks and side effects of magnetic and RF fields (for patients and staff) have been intensively examined for a long time, but there have been no long-term studies following persons who have been exposed to the static magnetic fields used in MRI. However, no permanent hazardous effects of a static magnetic field exposure upon human beings have yet been demonstrated.
Temporary possible side effects of high magnetic and RF fields:
•
Varying magnetic fields can induce so-called magnetic phosphenes that occur when an individual is subject to rapid changes of 2-5 T/s, which can produce a flashing sensation in the eyes. This temporary side effect does not seem to damage the eyes. Static field strengths used for clinical MRI examinations vary between 0.2 and 3.0 tesla;; field changes during the MRI scan vary in the dimension of mT/s. Experimental imaging units can use higher field strengths of up to 14.0 T, which are not approved for human use.
•
The Radio frequency pulses mainly produce heat, which is absorbed by the body tissue. If the power of the RF radiation is very high, the patient may be heated too much. To avoid this heating, the limit of RF exposure in MRI is up to the maximum specific absorption rate (SAR) of 4 W/kg whole body weight (can be different from country to country). For MRI safety reasons, the MRI machine starts no sequence, if the SAR limit is exceeded.
•
Very high static magnetic fields are needed to reduce the conductivity of nerves perceptibly. Augmentation of T waves is observed at fields used in standard imaging but this side effect in MRI is completely reversible upon removal from the magnet. Cardiac arrhythmia threshold is typically set to 7-10 tesla. The magnetohydrodynamic effect, which results from a voltage occurring across a vessel in a magnetic field and percolated by a saline solution such as blood, is irrelevant at the field strengths used.
The results of some animal and cellular studies suggest the possibility that electromagnetic fields may act as co-carcinogens or tumor promoters, but the data are inconclusive.
Up to 45 tesla, no important effects on enzyme systems have been observed. Neither changes in enzyme kinetics, nor orientation changes in macromolecules have been conclusively demonstrated.
There are some publications associating an increase in the incidence of leukemia with the location of buildings close to high-current power lines with extremely low-frequency (ELF) electromagnetic radiation of 50-60 Hz, and industrial exposure to electric and magnetic fields but a transposition of such effects to MRI or MRS seems unlikely.
Under consideration of the MRI safety guidelines, real dangers or risks of an exposure with common MRI field strengths up to 3 tesla as well as the RF exposure during the MRI scan, are not to be expected.
For more MRI safety information see also Nerve Conductivity,
Contraindications, Pregnancy
and Specific Absorption Rate.
See also the related poll result: ' In 2010 your scanner will probably work with a field strength of' | |  | |
• View the DATABASE results for 'MRI Risks' (9).
| | |
• View the NEWS results for 'MRI Risks' (3).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
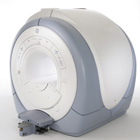
From GE Healthcare;
The Signa HDx MRI system is GE's leading edge whole body magnetic resonance scanner designed to support high resolution, high signal to noise ratio, and short scan times.
Signa HDx 3.0T offers new technologies like ultra-fast image reconstruction through the new XVRE recon engine, advancements in parallel imaging algorithms and the broadest range of premium applications. The HD applications, PROPELLER (high-quality brain imaging extremely resistant to motion artifacts), TRICKS (contrast-enhanced angiographic vascular lower leg imaging), VIBRANT (for breast MRI), LAVA (high resolution liver imaging with shorter breath holds and better organ coverage) and MR Echo (high-definition cardiac images in real time) offer unique capabilities.
Device Information and Specification CLINICAL APPLICATION Whole body
CONFIGURATION Compact short bore SE, IR, 2D/3D GRE, RF-spoiled GRE, 2DFGRE, 2DFSPGR, 3DFGRE, 3DFSPGR, 3DTOFGRE, 3DFSPGR, 2DFSE, 2DFSE-XL, 2DFSE-IR, T1-FLAIR, SSFSE, EPI, DW-EPI, BRAVO, Angiography: 2D/3D TOF, 2D/3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer H*W*D 240 x 2216,6 x 201,6 cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. | |  | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |