 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm '3 Tesla' found in 0 term [ ] and 11 definitions [ ] and 11 definitions [ ], (+ 11 Boolean[ ], (+ 11 Boolean[ ] results ] results
| previous 11 - 15 (of 22) nextResult Pages :  [1 2 3] [1 2 3]  [4 5] [4 5] |  | |  | Searchterm '3 Tesla' was also found in the following services: | | | | |
|  |  |
| |
|
(SAR) The Specific Absorption Rate is defined as the RF power absorbed per unit of mass of an object, and is measured in watts per kilogram (W/kg).
The SAR describes the potential for heating of the patient's tissue due to the application of the RF energy necessary to produce the MR signal. Inhomogeneity of the RF field leads to a local exposure where most of the absorbed energy is applied to one body region rather than the entire person, leading to the concept of a local SAR. Hot spots may occur in the exposed tissue, to avoid or at least minimize effects of such theoretical complications, the frequency and the power of the radio frequency irradiation should be kept at the lowest possible level. Averaging over the whole body leads to the global SAR.
It increases with field strength, radio frequency power and duty cycle, transmitter-coil type and body size. The doubling of the field strength from 1.5 Tesla (1.5T) to 3 Tesla ( 3T) leads to a quadrupling of SAR. In high and ultrahigh fields, some of the multiple echo, multiple-slice pulse sequences may create a higher SAR than recommended by the agencies. SAR can be reduced by lower flip angle and longer repetition times, which could potentially affect image contrast.
Normally no threatening increase in temperature could be shown. Even in high magnetic fields, the local temperature increases not more than 1°C. 2.1°C is the highest measured increase in skin temperature. Eddy currents may heat up implants and thus may cause local heating.
FDA SAR limits:
•
Whole body: 4W/kg/15-minute exposure averaged;
•
Head: 3W/kg/10-minute exposure averaged;
•
Head or torso: 8W/kg/5 minute exposure per gram of tissue;
•
Extremities: 12W/kg/5 minute exposure per gram of tissue.
IEC (International Electrotechnical Commission) SAR limits of some European countries:
All limits are averaged over 6 minutes.
•
Level 0 (normal operating mode): Whole body 2W/kg; Head 3.2W/kg; Head or Torso (local) 10W/kg;
Extremities (local) 20W/kg;
•
Level I (first level controlled operating mode): Whole body 4W/kg; Head 3.2W/kg; Head or Torso (local) 10W/kg; Extremities (local) 20W/kg;
•
Level II (second level controlled operating mode): All values are over Level I values.
(For more details: IEC 60601-2-33 (2002))
In most countries standard MRI systems are limited to a maximum SAR of 4 W/kg, so most scanning in level II is impossible.
For Level I, in addition to routine monitoring, particular caution must be exercised for patients who are sensitive to temperature increases or to RF energy.
For Japan different SAR limits are valid. | |  | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
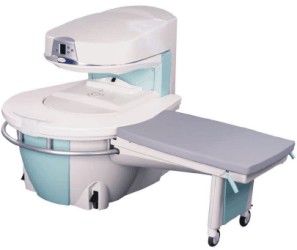
Manufactured by Esaote S.p.A.;
a low field open MRI scanner with permanent magnet for orthopedic use. The outstanding feature of this MRI system is a patient friendly design with 24 cm diameter, which allows the imaging of extremities and small body parts like shoulder MRI. The power consumption is around 1. 3 kW and the needed minimum floor space is an area of 16 sq m.
At RSNA 2006 Hologic Inc. introduced a new dedicated extremity MRI scanner, the Opera. Manufactured by Esaote is the Opera a redesign of Esaote's 0.2 Tesla E-Scan XQ platform, which now enables complete imaging of all extremities, including hip and shoulder applications. 'Real-time positioning' reportedly speeds patient setup and reduces exam times.
Esaote North America and Hologic Inc are the U.S. distributors of this MRI device.
Device Information and Specification CLINICAL APPLICATION Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE IMAGING MODES Single, multislice, volume study, fast scan, multi slab2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm 4096 gray lvls, 256 lvls in 3D POWER REQUIREMENTS 2,0 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'Opera (E-SCAN™ XQ)' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body Head and body coil standard; all other coils optional; open architecture makes system compatible with a wide selection of coils Standard: SE, IR, 2D/ 3D GRE and SPGR, Angiography;; 2D/ 3D TOF, 2D/ 3D Phase Contrast;; 2D/ 3D FSE, 2D/ 3D FGRE and FSPGR, SSFP, FLAIR, optional: EPI, 2D/ 3D Fiesta, FGRET, SpiralTR 4.4 msec to 12000 msec in increments of 1 msec TE 1.0 to 2000 msec; increments of 1 msec Simultaneous scan and reconstruction;; up to 100 images/second with Reflex 100 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode 0.08 mm; 0.02 mm optional POWER REQUIREMENTS 480 or 380/415 V Less than 0.0 3 L/hr liquid heliumSTRENGTH SmartSpeed 23 mT/m, HiSpeed Plus 33 mT/m 4.0 m x 2.8 m axial x radial | |  | |
• View the DATABASE results for 'Signa Infinity 1.0T™' (2).
| | | | |
|  |  | Searchterm '3 Tesla' was also found in the following services: | | | | |
|  |  |
| |
|
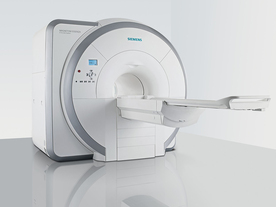
From Siemens Medical Systems;
Received FDA clearance in 2007.
The MAGNETOM Essenza is designed to combine high system performance with simple installation and power requirements to provide optimal operating costs for limited budgets. The standard system has up to 25 integrated coil elements and 8 independent radio frequency channels. Tim allows the combination of up to 4 different coils that reduce patient and coil repositioning.
The 1.5 Tesla system is designated for a complete range of clinical applications, including neurology, orthopedics, body imaging, angiography, cardiology, breast imaging, oncology and pediatric MRI.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, TMJ optional.
CHANNELS (min. / max. configuration)
8, 16
MAGNET WEIGHT (gantry included)
4350 kg in operation
DIMENSION H*W*D (gantry included)
145 x 226 x 216 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
30 mT/m, 300 msec to 10 mT/m
Passive, active; first order standard
second order optional
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 45 kVA
| |  | | | |
|  | |  |  |  |
| |
|
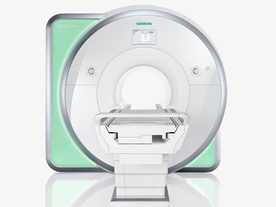
From Siemens Medical Systems;
Received FDA clearance in 2010.
The MAGNETOM Aera is a patient friendly, comfortable 1.5 Tesla MRI system with advanced radio frequency chain.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix + Day optimizing throughput), to enhance both productivity and image quality.
Tim 4G technology provides improved SNR. The standard system configuration of 48 radio frequency channels and 204 coil elements creates an imaging matrix that allows maximum use of coil elements at full field of view. Dot provides improved image consistency through new features like auto align, auto FoV and automatic bolus detection.
Device Information and Specification
CLINICAL APPLICATION
Whole body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder. Up to 60% more SNR with Tim 4G.
CHANNELS (min. / max. configuration)
48, 64
MINIMUM TE
3-D GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
3121 kg
DIMENSION H*W*D (gantry included)
145 x 231 x 219 cm
MAX. AMPLITUDE
33 or 45 mT/m
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 85 kVA
| |  | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |