 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Active Shimming' found in 1 term [ ] and 3 definitions [ ] and 3 definitions [ ], (+ 17 Boolean[ ], (+ 17 Boolean[ ] results ] results
| previous 16 - 20 (of 21) nextResult Pages :  [1] [1]  [2 3 4 5] [2 3 4 5] |  | | |  |  |  |
| |
|

From GE Healthcare;
three dedicated MRI systems - a neuro imager, a cardiovascular system, and a whole body scanner - all in one system.
Device Information and Specification CLINICAL APPLICATION Whole body Head and body coil standard; all other coils optional; open architecture makes system compatible with a wide selection of coils Optional 2D/3D brain and prostate Standard: SE, IR, 2D/3D GRE and SPGR, Angiography: 2D/3D TOF, 2D/3D Phase Contrast;; 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, FGRET, Spiral, TensorTR 1.2 to 12000 msec in increments of 1 msec TE 0.4 to 2000 msec in increments of 1 msec 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 Less than 0.03 L/hr liquid He STRENGTH Zoom 40 mT/m, whole body 23 mT/m 4.0 m x 2.8 m axial x radial | |  | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
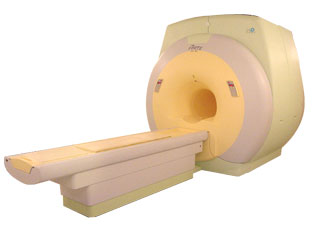
From ISOL Technology
'Ultra high field MR system, it's right close to you.
FORTE 3.0T is the new standard for the future ultra high field MR system.
If you are pushing the limits of your existing clinical MR scanner, the FORTE will surely take you to the next level of diagnostic imaging.
FORTE is the core leader of the medical technology in the 21st century. Proving effects of fMRI that cannot be measured with MRI less than 2.0T.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
128 x 128, 256 x 256, 512 x 512, 1024 x 1024
| |  | |
• View the DATABASE results for 'FORTE 3.0T™' (2).
| | | | |
|  | |  |  |  |
| |
|
Device Information and Specification CLINICAL APPLICATION Whole body SE, FE, IR, STIR, FFE, DEFFE, DESE, TSE, DETSE, Single shot SE, DRIVE, Balanced FFE, MRCP, Fluid Attenuated Inversion Recovery, Turbo FLAIR, IR-TSE, T1-STIR TSE, T2-STIR TSE, Diffusion Imaging, 3D SE, 3D FFE, Contrast Perfusion Analysis, MTC;; Angiography: CE-ANGIO, MRA 2D, 3D TOFOpen x 47 cm x infinite (side-first patient entry) POWER REQUIREMENTS 400/480 V | |  | |
• View the DATABASE results for 'Panorama 0.6T™' (2).
| | | | |
|  | |  |  |  |
| |
|
From Philips Medical Systems;
the Intera-family offers with this member a wide range of possibilities, efficiency and a ergonomic and intuitive serving-platform. Also available as Intera CV for cardiac and Intera I/T for interventional MR procedures.
The scanners are also equipped with SENSE technology, which is essential for high-quality contrast enhanced magnetic resonance angiography, inter active cardiac MR and diffusion tensor imaging ( DTI) fiber tracking.
The increased accuracy and clarity of MR scans obtained with this technology allow for faster and more accurate diagnosis of potential problems like patient friendliness and expands the breadth of applications including cardiology, oncology and interventional MR.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector; Optional SENSE coils: Flex-S-M-L, flex body, flex cardiac
SE, Modified-SE ( TSE), IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: PCA, MCA, Inflow MRA, CE
TR
2.9 (Omni), 1.6 (Power), 1.6 (Master/Expl) msec
TE
1.0 (Omni), 0.7 (Power), 0.5 (Master/Expl) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Pwr/Mstr/Expl)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for BOLD img.)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera 1.5T™' (2).
| | | | |
|  | |  |  |  |
| |
|

From Philips Medical Systems;
the Panorama 0.23 T, providing a new design optimized for patient comfort, faster reconstruction time than before (300 images/second) and new gradient
specifications. Philips' Panorama 0.23 T I/T supports MR-guided interventions, resulting in minimally invasive procedures, more targeted surgery, reduced recovery time and shorter hospital stays. Optional OptoGuide functionality enables real-time needle tracking. Philips' Panorama 0.23 TPanorama 0.2 R/T is the first and only open MRI system to enable radiation therapy planning using MR data sets. The Panorama also features the new and consistent Philips User Interface, an essential element of the Vequion clinical IT family of products and services.
Device Information and Specification CLINICAL APPLICATION Whole body SE, FE, IR, FFE, DEFFE, DESE, TSE, DETSE, Single shot SE, DRIVE, Balanced FFE, MRCP, Fluid Attenuated Inversion Recovery, Turbo FLAIR, IR-TSE, T1-STIR TSE, T2-STIR TSE, Diffusion Imaging, 3D SE, 3D FFE, MTC;; Angiography: CE-ANGIO, MRA 2D, 3D TOFOpen x 46 cm x infinite (side-first patient entry) POWER REQUIREMENTS 400/480 V COOLING SYSTEM TYPE Closed loop chilled water ( chiller included) | |  | |
• View the DATABASE results for 'Panorama 0.23T™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |