 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
Implants that involve magnets such as magnetic sphincters, stoma plugs, dental implants, etc., can be demagnetized by the MRI device. They should be removed prior to the examination.
A particular danger is presented by small metallic surgical implants. Haemostatic or other clips in the CNS can move in their position. Dislocation by magnetic attraction or torque presents a risk in MRI examinations. There is a minimal risk in other parts of the body, because after the healing phase of six to eight weeks, fibrosis and encasement of the clip help to keep it in a stable position.
The label stainless steel is not a guarantee for non-ferromagnetic steel.
See also Cardiac Pacemaker and MRI Safety. | |  | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
From Philips Medical Systems;
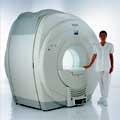
Philips Infinion 1.5 T is designed to maximize the efficiency and quality of patient care. Developed with the patient in mind, the Infinion is the shortest and most open 1.5T scanner available. The unique 'ultra short' 1.4 m magnet assures patient comfort and acceptance without compromising image quality and clinical performance.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Ultra short bore
Head, head / neck, integrated C-spine, L/T spine array, small large GP coils, body flex array, torso pelvis array, breast array, endocavitary, shoulder array, lower extremity, hand / wrist, cardiac, PV array
SE, TSE, SS TSE, EPI, IR, STIR, FLAIR, FFE, TFE, T1 TFE, T2 TFE, Presat, Fatsat, MTC, Diff-opt., Angiography: PCA, MCA, TOF
IMAGING MODES
Single slice, single volume, multi slice, multi volume
80 images/sec std.; up to320 opt.@256
H*W*D
233 (lead fitted) x 198 x 140 cm
POWER REQUIREMENTS
400/480 V
COOLING SYSTEM TYPE
Closed loop, chilled water
| |  | |
• View the DATABASE results for 'Infinion 1.5T™' (2).
| | | | |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector, Flex-S-M-L, flex body, flex cardiac, neuro-vascular, head
SE, Modified-SE ( TSE), DAVE, STIR, FLAIR, SPIR, MTC, Dynamic, Keyhole, CLEAR, Q Flow, Balanced FFE, Multi Chunk 3D, Multi Stack 3D, FFE-EPI, SE-EPI, IR-EPI, GRASE, Diffusion Imaging, Perfusion Imaging;; Angiography: Inflow MRA, TONE, PCA, CE MRA
RapidView Recon. greater than 500 @ 256 Matrix
128 x 128, 256 x 256,512 x 512,1024 x 1024
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera 0.5T™' (2).
| | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector, Optional SENSE Coils: Flex-S-M-L, Flex Body, Flex Cardiac
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
TR
Min. 2.9 (Omni) msec, 1.6 (Power) msec
TE
Min. 1.0 (Omni) msec, 0.7 (Power) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Power)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
STRENGTH
23 mT/m (Omni), 30 (Power) mT/m
| |  | |
• View the DATABASE results for 'Intera 1.0T™' (2).
| | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
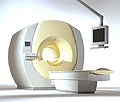
From Philips Medical Systems;
the Intera-family offers with this member a wide range of possibilities, efficiency and a ergonomic and intuitive serving-platform. Also available as Intera CV for cardiac and Intera I/T for interventional MR procedures.
The scanners are also equipped with SENSE technology, which is essential for high-quality contrast enhanced magnetic resonance angiography, interactive cardiac MR and diffusion tensor imaging ( DTI) fiber tracking.
The increased accuracy and clarity of MR scans obtained with this technology allow for faster and more accurate diagnosis of potential problems like patient friendliness and expands the breadth of applications including cardiology, oncology and interventional MR.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector; Optional SENSE coils: Flex-S-M-L, flex body, flex cardiac
SE, Modified-SE ( TSE), IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: PCA, MCA, Inflow MRA, CE
TR
2.9 (Omni), 1.6 (Power), 1.6 (Master/Expl) msec
TE
1.0 (Omni), 0.7 (Power), 0.5 (Master/Expl) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Pwr/Mstr/Expl)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for BOLD img.)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera 1.5T™' (2).
| | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |