 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
Efficiency characteristic of the device or material itself. | |  | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
The owner of MRI equipment has to ensure that the equipment does fulfill the local requirements.
In some countries, the requirements are more stringent than in others; in other countries, they are nonexistent.
The user in general is unable to check power output, gradient strength, or even field strength.
The manufacturer has to cover authorized hardware and software updates after the initial installation and has to give guarantee for the requirements.
Specially designed computer programs usually supervise the power output of MRI devices and will not allow or will interrupt any imaging or spectroscopy procedure exceeding those limits considered safe.
See also European Medicines Agency, FDA information:
www.fda.gov/cdrh/safety/mrisafety.html | |  | |
• View the DATABASE results for 'Legal Requirements' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
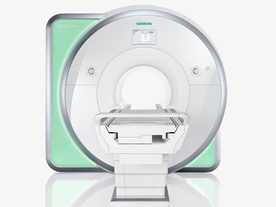
From Siemens Medical Systems;
Received FDA clearance in 2010.
The MAGNETOM Aera is a patient friendly, comfortable 1.5 Tesla MRI system with advanced radio frequency chain.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix + Day optimizing throughput), to enhance both productivity and image quality.
Tim 4G technology provides improved SNR. The standard system configuration of 48 radio frequency channels and 204 coil elements creates an imaging matrix that allows maximum use of coil elements at full field of view. Dot provides improved image consistency through new features like auto align, auto FoV and automatic bolus detection.
Device Information and Specification
CLINICAL APPLICATION
Whole body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder. Up to 60% more SNR with Tim 4G.
CHANNELS (min. / max. configuration)
48, 64
MINIMUM TE
3-D GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
3121 kg
DIMENSION H*W*D (gantry included)
145 x 231 x 219 cm
MAX. AMPLITUDE
33 or 45 mT/m
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 85 kVA
| |  | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
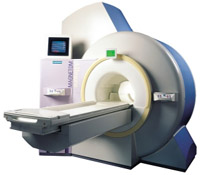
From Siemens Medical Systems;
the 3 T MAGNETOM Allegra is a dedicated MR headscanner, perfect as a research system in cognitive and neuroscience with MRS and fMRI. MAGNETOM Allegra is a full member of the MAGNETOM product family. It uses many common components, i.e. electronics, computer system, software and pulse sequence concepts.
Device Information and Specification
GRE, IR, FIR, STIR, TrueIR/FISP, FSE, FLAIR, MT, SS-FSE, MT-SE, MTC, MSE, EPI, GMR, fat/water sat./exc.
IMAGING MODES
Single, multislice, volume study, multi angle, multi oblique
178 images/sec at 256 x 256 at 100% FOV
1024 x 1024 full screen display
MAGNET WEIGHT (gantry included)
5500 kg
DIMENSION H*W*D (gantry included)
220 x 220 x 147 cm
POWER REQUIREMENTS
380/400/420/440/480 V
Passive, act.; 1st order std./2nd opt.
| |  | |
• View the DATABASE results for 'MAGNETOM Allegra™' (2).
| | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
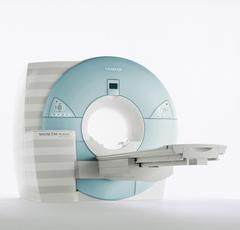
From Siemens Medical Systems;
MAGNETOM Avanto with Tim - Total imaging matrix technology.
For true whole-body anatomical coverage. For ultra-fast image
acquisition. Aids the physician in fast and precise
evaluation of systemic diseases like colon cancer, metastasis imaging, vessel diseases, and preventional exams. For claustrophobic patients,
MAGNETOM Avanto enables feetfirst exams for nearly all MR procedures. For obese patients, MAGNETOM Avanto supports up to 200 kg (400 lbs), without table movement restrictions. The AudioComfort technology enables up to a 30 dB(A) acoustic noise reduction, that means nearly all clinical routine sequences are running under 99 dB(A).
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Compact short bore
Body, Tim [32 x 8], Tim [76 x18], Tim [76 coil elements with up to 32 RF channels]
GRE, IR, FIR, STIR, TrueIR/FISP, FSE, FLAIR, MT, SS-FSE, MT-SE, MTC, MSE, EPI, 3D DESS//CISS/PSIF, GMR
IMAGING MODES
Single, multislice, volume study, multi angle, multi oblique
1024 x 1024 full screen display
POWER REQUIREMENTS
380/400/420/440/480 V
Passive, act.; 1st order std./2nd opt.
| |  | |
• View the DATABASE results for 'MAGNETOM Avanto™' (2).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |