 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
An undesirable background interference or disturbance that affects image quality.
The Noise is commonly characterized by the standard deviation of signal intensity in the image of a uniform object ( phantom) in the absence of artifacts. The measured noise may depend on the particular phantom used due to variable effects on the Q of the receiver coil. Noisy images appear when the SNR-Rate is too low - this is induced by the operator.
Image artifacts and RF noise can often be caused by the presence and/or operation of a medical device in the MR environment.
There are various noise sources in any electronic system, including Johnson noise, shot noise, thermal noise. Materials produce their own characteristic static magnetic field that can perturb the relationship between position and frequency essential to accurate image reconstruction.
RF noise, which often appears as static on the image, can be caused by a medical device located anywhere in the MR procedure room. RF noise is a result of excessive electromagnetic emissions from the medical device that interfere with the proper operation of the MR scanner. Since the MR procedure room is shielded from extraneous RF fields entering the room ( Faraday cage), operation of electromagnetically noisy equipment outside the room does not typically affect the MR scanner.
See Signal to Noise Ratio and Radio Frequency Noise Artifact. | |  | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
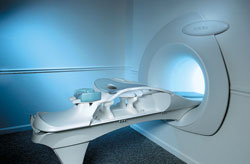
From Aurora Imaging Technology, Inc.;
The Aurora® 1.5T Dedicated Breast MRI System with Bilateral SpiralRODEO™ is the first and only FDA approved MRI device designed specifically for breast imaging. The Aurora System, which is already in clinical use at a growing number of leading breast care centers in the US, Europe, got in December 2006 also the approval from the State Food and Drug Administration of the People's Republic of China (SFDA).
'Some of the proprietary and distinguishing features of the Aurora System include: 1) an ellipsoid magnetic shim that provides coverage of both breasts, the chest wall and bilateral axillary lymph nodes; 2) a precision gradient coil with the high linearity required for high resolution spiral reconstruction;; 3) a patient-handling table that provides patient comfort and procedural utility; 4) a fully integrated Interventional System for MRI guided biopsy and localization; and 5) the user-friendly AuroraCAD™ computer-aided image display system designed to improve the accuracy and efficiency of diagnostic interpretations.'
Device Information and Specification
CONFIGURATION
Short bore compact
TE
From 5 ms for RODEO Plus to over 80 ms, 120 ms for T2 sequences
Around 0.02 sec for a 256x256 image, 12.4 sec for a 512 x 512 x 32 multislice set
20 - 36 cm, max. elliptical 36 x 44 cm
POWER REQUIREMENTS
150A/120V-208Y/3 Phase//60 Hz/5 Wire
| |  | |
• View the DATABASE results for 'Aurora® 1.5T Dedicated Breast MRI System' (2).
| | |
• View the NEWS results for 'Aurora® 1.5T Dedicated Breast MRI System' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
IMAGING MODES
Single, multislice, volume study, fast scan, multi slab
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240
| |  | |
• View the DATABASE results for 'C-SCAN™' (4).
| | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
A pacemaker is a device for internal or external battery-operated cardiac pacing to overcome cardiac arrhythmias or heart block. All implanted electronic devices are susceptible to the electromagnetic fields used in magnetic resonance imaging. Therefore, the main magnetic field, the gradient field, and the radio frequency (RF) field are potential hazards for cardiac pacemaker patients.
The pacemaker's susceptibility to static field and its critical role in life support have warranted special consideration. The static magnetic field applies force to magnetic materials. This force and torque effects rise linearly with the field strength of the MRI machines. Both, RF fields and pulsed gradients can induce voltages in circuits or on the pacing lead, which will heat up the tissue around e.g. the lead tip, with a potential risk of thermal injury.
Regulations for pacemakers provide that they have to switch to the magnet mode in static magnetic fields above 1.0 mT. In MR imaging, the gradient and RF fields may mimic signals from the heart with inhibition or fast pacing of the heart. In the magnet mode, most of the current pacemakers will pace with a fix pulse rate because they do not accept the heartsignals. However, the state of an implanted pacemaker will be unpredictable inside a strong magnetic field. Transcutaneous controller adjustment of pacing rate is a feature of many units. Some achieve this control using switches activated by the external application of a magnet to open/close the switch. Others use rotation of an external magnet to turn internal controls. The fringe field around the MRI magnet can activate such switches or controls. Such activations are a safety risk.
Areas with fields higher than 0.5 mT ( 5 Gauss Limit) commonly have restricted access and/or are posted as a safety risk to persons with pacemakers.

A Cardiac pacemaker is because the risks, under normal circumstances an absolute contraindication for MRI procedures.
Nevertheless, with special precaution the risks can be lowered. Reprogramming the pacemaker to an asynchronous mode with fix pacing rate or turning off will reduce the risk of fast pacing or inhibition. Reducing the SAR value reduces the potential MRI risks of heating. For MRI scans of the head and the lower extremities, tissue heating also seems to be a smaller problem. If a transmit receive coil is used to scan the head or the feet, the cardiac pacemaker is outside the sending coil and possible heating is very limited. | |  | |
• View the DATABASE results for 'Cardiac Pacemaker' (6).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
Contact Information
MAIL
Food and Drug Administration
5600 Fishers Lane
Rockville, Maryland 20857
USA
PHONE
+1-888-463-6332 (see also under E-MAIL)
| |  | |
• View the DATABASE results for 'Food and Drug Administration' (9).
| | |
• View the NEWS results for 'Food and Drug Administration' (20).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |