 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
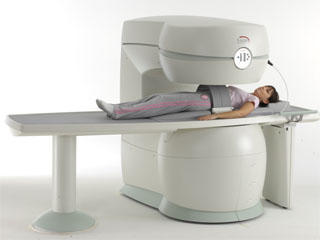
From Esaote S.p.A.;
Esaote introduced the S-SCAN at RSNA in November 2007. The S-SCAN is a dedicated joint and spine MR scanner derived from the company's earlier G-SCAN system. Unlike the G-SCAN, neither the patient table nor
the magnet can rotate from horizontal to vertical position. The patient table can only moved manually. Improved electronics, new coils for lumbar and cervical spine, new pulse sequences, a modified version of the magnet poles and gradient coils are used with a new software release in the S-SCAN.
Esaote North America is the exclusive U.S. distributor of this MRI device.
Device Information and Specification SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE POWER REQUIREMENTS 3 kW; 110/220 V single phase | |  | |
• View the NEWS results for 'S-SCAN' (1).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|

From GE Healthcare;
GE Healthcare has added the Signa HDe 1.5T™, a compact MRI device at an affordable price to its family of MRI products. It has a single electronic cabinet that can be positioned inside the scanner room rather than
in a separate equipment room. The Signa HDe 1.5T can be installed in the same physical location as 0.5T MRI systems with minimal construction costs. According to GE, the installation has been simplified to last only 7 days and has a 30 percent smaller footprint than a typical 1.5T system.
The 1.5T Signa™ HDe MRI system is substantially equivalent to the currently marketed GE 1.5T machines. The data acquisition system supports 1, 4, 8 independent receive channels and multiple independent coil elements per channel during a single acquisition series. The gradient specifications of HDe are lower than other GE Signa 1.5T MRI systems, but it can support clinical applications in cardiac and spectroscopy imaging.
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Compact short bore 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 less than 0.03 L/hr liquid helium | |  | |
• View the NEWS results for 'Signa HDe 1.5T™' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
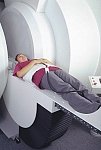
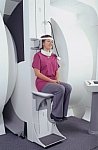
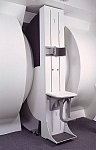
From FONAR Corporation;
in October of 2004, the company changed the product name of the Stand-Up™ MRI to the Upright™ MRI. The Indomitable™, Upright™ MRI is the only open MRI in the world that can perform positional MRI (pMRI), i.e. the Upright MRI™ scans patients in upright, weight-bearing positions, in addition to the conventional lie-down positions. The Upright™ MRI is the only device that can scan patients in the position their symptoms occur, in their position of pain. In early clinical reports independently confirm the effectiveness and potential of positional MRI. In October 2000, Fonar received permission to market the Indomitable™ from the FDA.
Device Information and Specification CLINICAL APPLICATION Whole body -
weight-bearing MRI -
position imaging (flexion, extension, bending, standing, sitting and recumbent scanning)
CONFIGURATION Front-open and Top-open MRIPOWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Water, closed-loop | |  | |
• View the DATABASE results for 'Upright™ MRI' (5).
| | |
• View the NEWS results for 'Upright™ MRI' (9).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|

From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0.3 T field-strength magnet and phased array coils deliver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
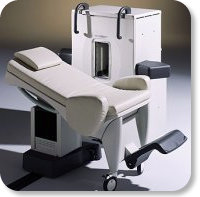
Developed by GE Lunar; the ARTOSCAN™-M is designed specifically for in-office musculoskeletal imaging. ARTOSCAN-M's compact, modular design allows placing within a clinical environment, bringing MRI to the patient. Patients remain outside the magnet at all times during the examinations, enabling constant patient-technologist contact. ARTOSCAN-M requires no special RF room, magnetic shielding, special power supply or air conditioning.
The C-SCAN™ (also known as Artoscan C) is developed from the ARTOSCAN™ - M, with a new computer platform.
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
SLICE THICKNESS
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240V
| |  | |
• View the DATABASE results for 'ARTOSCAN™ - M' (3).
| | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |