 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
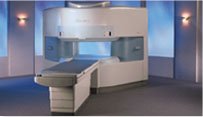
From Hitachi Medical Systems America, Inc.;
the AIRIS made its debut in 1995. Hitachi followed up with the AIRIS II system, which has proven equally successfully. 'All told, Hitachi has installed more than 1,000 MRI systems in the U.S., holding more than 17 percent of the total U.S. MRI installed base, and more than half of the installed base of open MR systems,' says Antonio Garcia, Frost and Sullivan industry research analyst.
Now Altaire employs a blend of innovative Hitachi features called VOSIâ„¢ technology, optimizing each sub-system's performance in concert with the
other sub-systems, to give the seamless mix of high-field performance
and the patient comfort, especially for claustrophobic patients, of open MR systems.
Device Information and Specification
CLINICAL APPLICATION
Whole body
DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP)
SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 3.6 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 8 - 250msec IR: 5.2 -7,680msec GE: 1.8 - 2,000 msec FSE: 5.2 - 7,680
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
COOLING SYSTEM TYPE
Water-cooled
3.1 m lateral, 3.6 m vertical
| |  | | | | | | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
| |  | |
• View the DATABASE results for 'Antenna' (8).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
An image artifact is a structure not normally present but visible as a result of a limitation or malfunction in the hardware or software of the MRI device, or in other cases a consequence of environmental influences as heat or humidity or it can be caused by the human body (blood flow, implants etc.). The knowledge of MRI artifacts (brit. artefacts) and noise producing factors is important for continuing maintenance of high image quality. Artifacts may be very noticeable or just a few pixels out of balance but can give confusing artifactual appearances with pathology that may be misdiagnosed.
Changes in patient position, different pulse sequences, metallic artifacts, or other imaging variables can cause image distortions, which can be reduced by the operator; artifacts due to the MR system may require a service engineer.
Many types of artifacts may occur in magnetic resonance imaging. Artifacts in magnetic resonance imaging are typically classified as to their basic principles, e.g.:
•
Physiologic (motion, flow)
•
Hardware (electromagnetic spikes, ringing)
Several techniques are developed to reduce these artifacts (e.g. respiratory compensation, cardiac gating, eddy current compensation) but sometimes these effects can also be exploited, e.g. for flow measurements.
See also the related poll result: ' Most outages of your scanning system are caused by failure of'
| |  | |
• View the DATABASE results for 'Artifact' (166).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
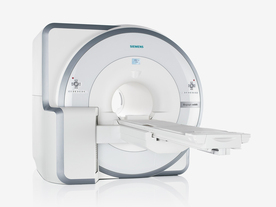
FDA cleared and CE Mark 2011.
The Biograph mMR has a fully-integrated design for simultaneous PET/MRI imaging. The dedicated hardware includes solid-state, avalanche photodiode PET detector and adapted, PET-compatible MR coils.
The possibility of truly simultaneous operation allows the acquisition of several magnetic resonance imaging ( MRI) sequences during the positron emission tomography (PET) scan, without increasing the examination time.
See also Hybrid Imaging.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Simultaneous PET/MRI
26 cm (typical overlap 23%)
A-P 45, R-L 50, H-F 50 cm
PET RING DIAMETER
65.6 cm
PATIENT SCAN RANGE
199 cm
HORIZONTAL SPEED
200 mmsec
PET DETECTOR
Solid state, 4032 avalanche photo diodes
DETECTOR SCINTILLATION MATERIAL
LSO, 28672 crystals
CRYSTAL SIZE
4 x 4 x 20 mm
DIMENSION H*W*D (gantry included)
335 x 230 x 242 cm (finshed covers)
COOLING SYSTEM
PET system: water; MRI system: water
Aautomatic, patient specific shim; active shim 3 linear and 5 non-linear channels (seond order)
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; Total system 110kW
| |  | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
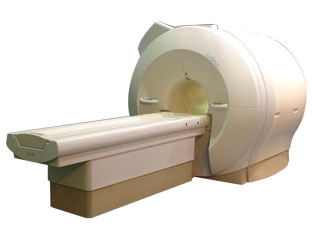
'Next generation MRI system 1.5T CHORUS developed by ISOL Technology is optimized for both clinical diagnostic imaging and for research development.
CHORUS offers the complete range of feature oriented advanced imaging techniques- for both clinical routine and research. The compact short bore magnet, the patient friendly design and the gradient technology make the innovation to new degree of perfection in magnetic resonance.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
Spin Echo, Gradient Echo, Fast Spin Echo,
Inversion Recovery ( STIR, Fluid Attenuated Inversion Recovery), FLASH, FISP, PSIF, Turbo Flash ( MPRAGE ),TOF MR Angiography, Standard echo planar imaging package (SE-EPI, GE-EPI), Optional:
Advanced P.A. Imaging Package (up to 4 ch.), Advanced echo planar imaging package,
Single Shot and Diffusion Weighted EPI, IR/FLAIR EPI
STRENGTH
20 mT/m (Upto 27 mT/m)
| |  | |
• View the DATABASE results for 'CHORUS 1.5T™' (2).
| | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |