 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
Another term for detector, by analogy to broadcast radio receivers. Other terms used for devices of this type include modulators, phase sensitive detector and mixers. | |  | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  | |  | |  |  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
E-Z-EM, Inc. is headquartered in New York and develops, manufactures and markets diagnostic imaging products used by radiologists like the MRI bowel marker Gadolite® Oral Suspension.
In addition to medical diagnostics systems, the company's products include MR-compatible interventional devices, such as biopsy needles. E-Z-EM is also a third-party contract manufacturer of contrast media.
Bracco Diagnostics, Inc. , the US-based subsidiary of Bracco Imaging S.p.A. and part of the Bracco Group, announced in Oct. 2007 that it has entered into a merger agreement to acquire E-Z-EM, Inc. (NASDAQ: EZEM) for a total consideration of about $ 240 million. Upon completion of the procedure and subject to the approval of the regulatory authorities, Bracco Diagnostics Inc. will incorporate E-Z-EM.
Contact Information
MAIL
E-Z-EM, Inc.
Westbury, New York
USA
| |  | |
• View the NEWS results for 'E-Z-EM, Inc.' (1).
| | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
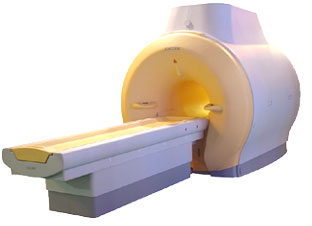
'MRI system is not an expensive equipment anymore.
ENCORE developed by ISOL Technology is a low cost MRI system with the advantages like of the 1.0T MRI scanner. Developed specially for the overseas market, the ENCORE is gaining popularity in the domestic market by medium sized hospitals.
Due to the optimum RF and Gradient application technology. ENCORE enables to obtain high resolution imaging and 2D/3D Angio images which was only possible in high field MR systems.'
- Less consumption of the helium gas due to the ultra-lightweight magnet specially designed and manufactured for ISOL.
- Cost efficiency MR system due to air cooling type (equivalent to permanent magnetic).
- Patient processing speed of less than 20 minutes.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
| |  | |
• View the DATABASE results for 'ENCORE 0.5T™' (2).
| | | | |
|  |  | Searchterm 'Device' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
SE, IR, FSE, FIR, GE, SG, BASG, PBSG, PCIR, DWI, Radial, Angiography: TOF, FLUTE (Fluoro-triggered bolus MRA), Time-resolved MRA
IMAGING MODES
Single, multislice, volume study
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
| |  | |
• View the DATABASE results for 'Echelon™ 1.5T' (2).
| | |
• View the NEWS results for 'Echelon™ 1.5T' (3).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |