 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Fringe Field' found in 3 terms [ ] and 62 definitions [ ] and 62 definitions [ ] ]
| previous 31 - 35 (of 65) nextResult Pages :  [1] [1]  [2 3 4 5 6 7 8 9 10 11 12 13] [2 3 4 5 6 7 8 9 10 11 12 13] |  | | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
GRE, IR, FIR, STIR, TrueIR/FISP, FSE, FLAIR, MT, SS-FSE, MT-SE, MTC, MSE, EPI, GMR, fat/water sat./exc.
IMAGING MODES
Single, multislice, volume study, multi angle, multi oblique
TR
2.4 msec std.; 2.0 opt.; 1.8 w/ 30mT/m at 256matrix
TE
1.1 msec std.; 0.9 opt.; 0.78 w/30 mT/m at 256matrix
178 images/sec at 256 x 256 at 100% FOV
1024 x 1024 full screen display
21 micrometer in plane, 11 micrometer optional
MAGNET WEIGHT (gantry included)
3500kg, 5000kg in operation
DIMENSION H*W*D (gantry included)
POWER REQUIREMENTS
380/400/420/440/480 V
STRENGTH
20/35 mT/m standard, 30/52 opt.
Passive, act.; 1st order std./2nd opt.
| |  | | | | | | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
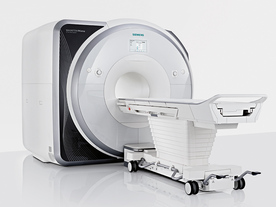
From Siemens Medical Systems;
Received FDA clearance in 2013.
The MAGNETOM Prisma is the 3T PowerPack for exploration that offers most demanding clinical and research challenges of today and the future. The latest parallel transmit technology, TimTX TrueShape, enables zooming into specific body regions for enhanced image quality. Furthermore, the Tim 4G integrated coil technology offers remarkable imaging flexibility and supports complex
examinations across the whole body.
Onsite upgrades to the MAGNETOM Prisma for customers who have already installed the 3 Tesla MAGNETOM Trio are possible.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
64, 128
MAGNET WEIGHT (gantry included)
13000 kg
DIMENSION H*W*D (gantry included)
173 x 230 x 222 cm
Passive, active; first order,
second order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground;
| |  | | | |
|  | |  |  |  |
| |
|
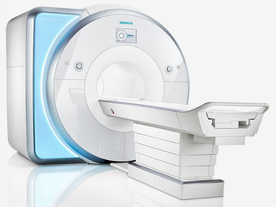
From Siemens Medical Systems;
Received FDA clearance in 2010.
MAGNETOM Skyra is a top-of-the-line, patient friendly wide bore 3 Tesla MRI system.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix and Day optimizing throughput), to enhance both productivity and image quality with the complete range of advanced applications for clinical routine and research. Tim 4G features lighter, trimmer MRI coils that take up less space inside the magnet but deliver a high coil element density with increased signal to noise ratio and the possibility to use high iPAT factors.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder.
CHANNELS (min. / max. configuration)
48, 64, 128
Chemical shift imaging, single voxel spectroscopy
MINIMUM TE
3D T1 spoiled GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
5768 kg
DIMENSION H*W*D (gantry included)
173 x 231 x 219 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 110 kVA
| |  | | | |
|  | |  |  |  |
| |
|
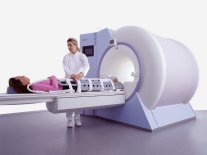
From Siemens Medical Systems;
while older navigator techniques take up to 40 minutes to create, the high performance of the MAGNETOM Sonata system enables 'complete examinations in less than 15 minutes'. It creates a new standard of diagnostic confidence and moves Cardiac MR from the research setting into routine clinical practice.
Device Information and Specification CLINICAL APPLICATION Whole body Body, head, spine, knee, neck, TMJ, extremity, head, breast, shoulder, others GRE, IR, FIR, STIR, TrueIR/FISP, FSE, FLAIR, MT, SS-FSE, MT-SE, MTC, MSE, EPI, 3D DESS//CISS/PSIF, GMR IMAGING MODES Single, multislice, volume study, multi angle, multi oblique178 images/sec at 256 x 256 at 100% FOV1024 x 1024 full screen display 4050kg, 5500kg in operation POWER REQUIREMENTS 380/400/420/440/480 V Passive, act.; 1st order std./2nd opt. | |  | |
• View the DATABASE results for 'MAGNETOM Sonata™' (2).
| | | | |
|  | |  |  |  |
| |
|
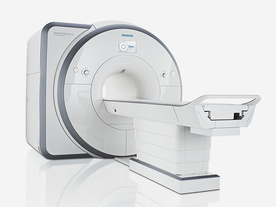
From Siemens Medical Systems;
Received FDA clearance in 2012.
The MAGNETOM Spectra is a cost-optimized high field MRI system with Tim 4G and Dot technologies. The system consumes less energy compared to other 3 Tesla scanners. The magnet-cooling helium is contained in a closed loop, which prevents the gas from escaping and reduces the need for refills. TimTX includes innovative techniques in the radio frequency excitation hardware as well as new application and processing features enabling uniform RF distribution in all body regions.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
Head, spine, torso/ body coil, neurovascular, neck and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, endorectal optional.
Chemical shift imaging, single voxel spectroscopy
DIMENSION H*W*D (gantry included)
173 x 231 x 219 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
Passive, active; first order standard, second order optional
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; connection value with chiller 100 kvA /without chiller 60 kVA
| |  | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |