 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Gating' found in 4 terms [ ] and 63 definitions [ ] and 63 definitions [ ] ]
| previous 26 - 30 (of 67) nextResult Pages :  [1] [1]  [2 3 4 5 6 7 8 9 10 11 12 13 14] [2 3 4 5 6 7 8 9 10 11 12 13 14] |  | |  | Searchterm 'Gating' was also found in the following services: | | | | |
|  |  |
| |
|
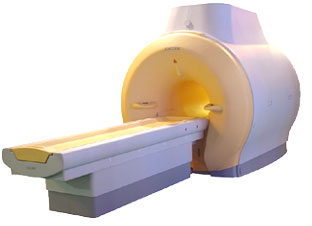
'MRI system is not an expensive equipment anymore.
ENCORE developed by ISOL Technology is a low cost MRI system with the advantages like of the 1.0T MRI scanner. Developed specially for the overseas market, the ENCORE is gaining popularity in the domestic market by medium sized hospitals.
Due to the optimum RF and Gradient application technology. ENCORE enables to obtain high resolution imaging and 2D/3D Angio images which was only possible in high field MR systems.'
- Less consumption of the helium gas due to the ultra-lightweight magnet specially designed and manufactured for ISOL.
- Cost efficiency MR system due to air cooling type (equivalent to permanent magnetic).
- Patient processing speed of less than 20 minutes.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
| |  | | | | | | | | |
|  |  | Searchterm 'Gating' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
Opt. (WIP) Single and Multi Voxel
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 1.2 msec min. FE
less than 0.015 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
4050 kg (bare magnet incl. L-He)
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart AG™ with Pianissimo' (2).
| | | | |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 0.9 msec min. FE
less than 0.011 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
4050 kg (bare magnet incl. L-He)
POWER REQUIREMENTS
380/400/415/440/480 V
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart XG™ with Pianissimo' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Gating' was also found in the following services: | | | | |
|  |  |
| |
|
From Toshiba America Medical Systems Inc.;
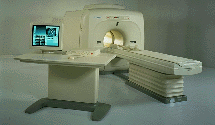
FLEXART™ series is a 0.5 T superconducting MRI system that has been designed to meet the expanding role of MRI in today's clinical environment. The system utilizes innovative technologies such as digital RF, high speed actively shielded gradients and optimized RF coils which support a wide range of MRI developments.
Device Information and Specification
CLINICAL APPLICATION
Whole body
Quadrature, solenoid and multi-channel configurations
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
POWER REQUIREMENTS
380/400/415/440/480 V
COOLING SYSTEM TYPE
Closed-loop water-cooled
| |  | |
• View the DATABASE results for 'FLEXART™' (2).
| | | | |
|  |  | Searchterm 'Gating' was also found in the following services: | | | | |
|  |  |
| |
|
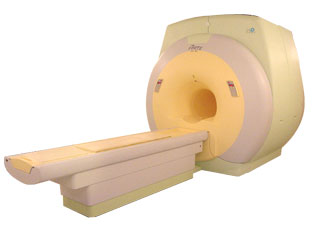
From ISOL Technology
'Ultra high field MR system, it's right close to you.
FORTE 3.0T is the new standard for the future ultra high field MR system.
If you are pushing the limits of your existing clinical MR scanner, the FORTE will surely take you to the next level of diagnostic imaging.
FORTE is the core leader of the medical technology in the 21st century. Proving effects of fMRI that cannot be measured with MRI less than 2.0T.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
128 x 128, 256 x 256, 512 x 512, 1024 x 1024
| |  | |
• View the DATABASE results for 'FORTE 3.0T™' (2).
| | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |