 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Gauss' was also found in the following services: | | | | |
|  |  |
| |
|
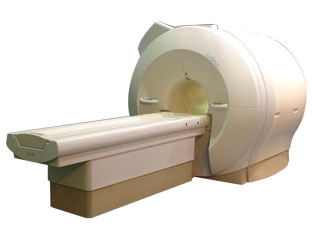
'Next generation MRI system 1.5T CHORUS developed by ISOL Technology is optimized for both clinical diagnostic imaging and for research development.
CHORUS offers the complete range of feature oriented advanced imaging techniques- for both clinical routine and research. The compact short bore magnet, the patient friendly design and the gradient technology make the innovation to new degree of perfection in magnetic resonance.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
Spin Echo, Gradient Echo, Fast Spin Echo,
Inversion Recovery ( STIR, Fluid Attenuated Inversion Recovery), FLASH, FISP, PSIF, Turbo Flash ( MPRAGE ),TOF MR Angiography, Standard echo planar imaging package (SE-EPI, GE-EPI), Optional:
Advanced P.A. Imaging Package (up to 4 ch.), Advanced echo planar imaging package,
Single Shot and Diffusion Weighted EPI, IR/FLAIR EPI
STRENGTH
20 mT/m (Upto 27 mT/m)
| |  | | | |
|  |  | Searchterm 'Gauss' was also found in the following service: | | | | |
|  |  |
| |
|
A pacemaker is a device for internal or external battery-operated cardiac pacing to overcome cardiac arrhythmias or heart block. All implanted electronic devices are susceptible to the electromagnetic fields used in magnetic resonance imaging. Therefore, the main magnetic field, the gradient field, and the radio frequency (RF) field are potential hazards for cardiac pacemaker patients.
The pacemaker's susceptibility to static field and its critical role in life support have warranted special consideration. The static magnetic field applies force to magnetic materials. This force and torque effects rise linearly with the field strength of the MRI machines. Both, RF fields and pulsed gradients can induce voltages in circuits or on the pacing lead, which will heat up the tissue around e.g. the lead tip, with a potential risk of thermal injury.
Regulations for pacemakers provide that they have to switch to the magnet mode in static magnetic fields above 1.0 mT. In MR imaging, the gradient and RF fields may mimic signals from the heart with inhibition or fast pacing of the heart. In the magnet mode, most of the current pacemakers will pace with a fix pulse rate because they do not accept the heartsignals. However, the state of an implanted pacemaker will be unpredictable inside a strong magnetic field. Transcutaneous controller adjustment of pacing rate is a feature of many units. Some achieve this control using switches activated by the external application of a magnet to open/close the switch. Others use rotation of an external magnet to turn internal controls. The fringe field around the MRI magnet can activate such switches or controls. Such activations are a safety risk.
Areas with fields higher than 0.5 mT ( 5 Gauss Limit) commonly have restricted access and/or are posted as a safety risk to persons with pacemakers.

A Cardiac pacemaker is because the risks, under normal circumstances an absolute contraindication for MRI procedures.
Nevertheless, with special precaution the risks can be lowered. Reprogramming the pacemaker to an asynchronous mode with fix pacing rate or turning off will reduce the risk of fast pacing or inhibition. Reducing the SAR value reduces the potential MRI risks of heating. For MRI scans of the head and the lower extremities, tissue heating also seems to be a smaller problem. If a transmit receive coil is used to scan the head or the feet, the cardiac pacemaker is outside the sending coil and possible heating is very limited. | |  | |
• View the DATABASE results for 'Cardiac Pacemaker' (6).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
The principal contraindications of the MRI procedure are mostly related to the presence of metallic implants in a patient. The risks of MRI scans increase with the used field strength. In general, implants are becoming increasingly MR safe and an individual evaluation is carried out for each case.

Some patients should not be examined in MRI machines, or come closer than the 5 Gauss line to the system.
Absolute Contraindications for the MRI scan:
•
electronically, magnetically, and mechanically activated implants
•
metallic splinters in the eye
Patients with absolute contraindications should not be examined or only with special MRI safety precautions. Patients with an implanted cardiac pacemaker have been scanned on rare occasions, but pacemakers are generally considered an absolute contraindication. Relative contraindications may pose a relative hazard, and the type and location of an implant should be assessed prior to the MRI examination.
Relative Contraindications for the MRI scan:
•
other pacemakers, e.g. for the carotid sinus
Osteosynthesis material is usually anchored so well in the patients that no untoward effect will result. Another effect on metal parts in the patient's body is the heating of these parts through induction. In addition, image quality may be severely degraded. The presence of other metallic implants such as surgical clips etc. should be made known to the MRI operators. Most of these materials are non-magnetic, but if magnetic, they can pose a hazard.
See also MRI safety, Pregnancy, Claustrophobia and Tattoos. | | | | | | | | |
• View the DATABASE results for 'Contraindications' (11).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Gauss' was also found in the following services: | | | | |
|  |  |
| |
|
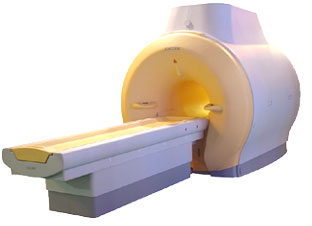
'MRI system is not an expensive equipment anymore.
ENCORE developed by ISOL Technology is a low cost MRI system with the advantages like of the 1.0T MRI scanner. Developed specially for the overseas market, the ENCORE is gaining popularity in the domestic market by medium sized hospitals.
Due to the optimum RF and Gradient application technology. ENCORE enables to obtain high resolution imaging and 2D/3D Angio images which was only possible in high field MR systems.'
- Less consumption of the helium gas due to the ultra-lightweight magnet specially designed and manufactured for ISOL.
- Cost efficiency MR system due to air cooling type (equivalent to permanent magnetic).
- Patient processing speed of less than 20 minutes.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
| |  | |
• View the DATABASE results for 'ENCORE 0.5T™' (2).
| | | | |
|  |  | Searchterm 'Gauss' was also found in the following service: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Cylindrical Wide Short Bore
Opt. (WIP) Single and Multi Voxel
SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC
IMAGING MODES
Single, multislice, volume study
TE
8 msec min. SE; 1.2 msec min. FE
less than 0.015 (256x256)
1.0 min. 2-DFT: 0.2 min. 3-DFT
32-1024, phase;; 64-1024, freq.
65.5 cm, patient aperture
4050 kg (bare magnet incl. L-He)
COOLING SYSTEM TYPE
Closed-loop water-cooled
Liquid helium: approx. less than 0.05 L/hr
Passive, active, auto-active
| |  | |
• View the DATABASE results for 'Excelart AG™ with Pianissimo' (2).
| | | | |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |