 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Magnetic Gradient' found in 1 term [ ] and 4 definitions [ ] and 4 definitions [ ], (+ 19 Boolean[ ], (+ 19 Boolean[ ] results ] results
| previous 6 - 10 (of 24) nextResult Pages :  [1] [1]  [2 3 4 5] [2 3 4 5] |  | |  | Searchterm 'Magnetic Gradient' was also found in the following services: | | | | |
|  |  |
| |
|
A small linear magnetic field applied in addition to (superimposed on) the large static magnetic field in a MRI scanner. The strength ( amplitude) and direction of the gradient fields change during the scan, which allows each small volume element ( voxel) within the imaging volume to resonate at a different frequency. In this way, spatial encoding may be performed. | |  | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
Gx, Gy, Gz - conventional symbols for gradient magnetic field. x, y, z denote spatial direction component of gradient, i.e. direction along that the field changes. | |  | | | |
|  | |  |  |  |
| |
|
| |  | |
• View the DATABASE results for 'Magnetic Field Gradient' (28).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | Searchterm 'Magnetic Gradient' was also found in the following services: | | | | |
|  |  |
| |
|
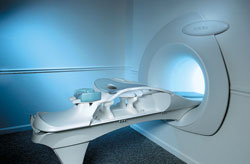
From Aurora Imaging Technology, Inc.;
The Aurora® 1.5T Dedicated Breast MRI System with Bilateral SpiralRODEO™ is the first and only FDA approved MRI device designed specifically for breast imaging. The Aurora System, which is already in clinical use at a growing number of leading breast care centers in the US, Europe, got in December 2006 also the approval from the State Food and Drug Administration of the People's Republic of China (SFDA).
'Some of the proprietary and distinguishing features of the Aurora System include: 1) an ellipsoid magnetic shim that provides coverage of both breasts, the chest wall and bilateral axillary lymph nodes; 2) a precision gradient coil with the high linearity required for high resolution spiral reconstruction;; 3) a patient-handling table that provides patient comfort and procedural utility; 4) a fully integrated Interventional System for MRI guided biopsy and localization; and 5) the user-friendly AuroraCAD™ computer-aided image display system designed to improve the accuracy and efficiency of diagnostic interpretations.'
Device Information and Specification
CONFIGURATION
Short bore compact
TE
From 5 ms for RODEO Plus to over 80 ms, 120 ms for T2 sequences
Around 0.02 sec for a 256x256 image, 12.4 sec for a 512 x 512 x 32 multislice set
20 - 36 cm, max. elliptical 36 x 44 cm
POWER REQUIREMENTS
150A/120V-208Y/3 Phase//60 Hz/5 Wire
| |  | |
• View the DATABASE results for 'Aurora® 1.5T Dedicated Breast MRI System' (2).
| | |
• View the NEWS results for 'Aurora® 1.5T Dedicated Breast MRI System' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|

If a device is to be labeled MR Safe, the following information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment (i.e. the MRI scan room) it does not pose an increased safety risk to the patient or other personnel,
•
a scientifically-based rationale for why data are not necessary to prove the safety of the device in the MR environment (for example, a passive device made entirely of a polymer known to be nonreactive in strong magnetic fields).
If a device is to be labeled MR Compatible, the following information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment, it is MR safe that it performs its intended function without performance degradation, and that it does not adversely affect the function of the MRI scanner (e.g. no significant image artifacts or noise). Any image artifact or noise due to the medical device should be quantified (e.g., % volume affected, signal to noise ratio),
•
a scientifically-based rationale for why data are not necessary to prove the compatibility of the device in the MRI environment.
Test Conditions:
The static magnetic field strength ( Gauss (G) or Tesla (T)) to which the device was tested and demonstrated to be MRI 'safe', 'compatible', or 'intended for use in' should be related to typical machine ratings (e.g. 0.5 T, 1.5 T, 2.0 T, and shielded or unshielded magnet, etc).
The same conditions should be used for the spatial gradient ( field strength per unit distance (i.e., G/cm)) in which the device was tested and demonstrated to be 'safe', 'compatible', or 'intended for use in'.
Also the RF transmitter power used during testing of the device, should be related to this typical machine ratings. | |  | |
• View the DATABASE results for 'MR Compatibility' (4).
| | |
• View the NEWS results for 'MR Compatibility' (2).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |