 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | 'Magnetic Resonance Imaging' | |
Result : Searchterm 'Magnetic Resonance Imaging' found in 5 terms [ ] and 64 definitions [ ] and 64 definitions [ ] ]
| previous 16 - 20 (of 69) nextResult Pages :  [1] [1]  [2 3 4 5 6 7 8 9 10 11 12 13 14] [2 3 4 5 6 7 8 9 10 11 12 13 14] |  | |  | Searchterm 'Magnetic Resonance Imaging' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Mobile compact
Whole body, intra-operative head, neck volume, atlas head//neck vascular quadrature phased array, spine quadrature, C/T/L spine phased array, small joint, large joint, TMJ bilateral, shoulder phased array, extremity quadrature volume, wrist, hand quadrature, general purpose flexible, pelvis/abdomen phased array, body quadrature, phased array flexible, breast bilateral
IMAGING MODES
Localizer, single slice, multislice, volume
| |  | | | |
|  | |  |  |  |
| |
|
| | | | | | | | |
• View the DATABASE results for 'Adverse Reaction' (8).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
GE Medical Systems and Amersham announced in April 2004 the completion of a share exchange acquisition of Amersham Health by GE. The result of this acquisition is the new GE Healthcare, based in the UK, totally owned by General Electric (GE).
Amersham plc, was a producer of contrast imaging agents used to enhance image quality in X-ray, magnetic resonance imaging, and ultrasound procedures. It was also a leading producer of radiopharmaceuticals used in nuclear medicine imaging. Amersham Health was the firm's imaging, diagnostics, and therapeutics segment. Amersham plc was involved in biotechnology research through its Amersham Biosciences unit, which made scanners, sequencers, microarrays, industrial separations, and other research supplies.
| |  | |
• View the DATABASE results for 'Amersham plc' (3).
| | |
• View the NEWS results for 'Amersham plc' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Magnetic Resonance Imaging' was also found in the following services: | | | | |
|  | |  | |  |  |  |
| |
|
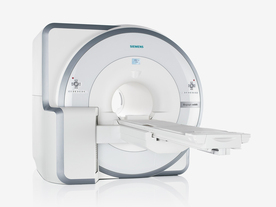
FDA cleared and CE Mark 2011.
The Biograph mMR has a fully-integrated design for simultaneous PET/MRI imaging. The dedicated hardware includes solid-state, avalanche photodiode PET detector and adapted, PET-compatible MR coils.
The possibility of truly simultaneous operation allows the acquisition of several magnetic resonance imaging ( MRI) sequences during the positron emission tomography (PET) scan, without increasing the examination time.
See also Hybrid Imaging.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Simultaneous PET/MRI
26 cm (typical overlap 23%)
A-P 45, R-L 50, H-F 50 cm
PET RING DIAMETER
65.6 cm
PATIENT SCAN RANGE
199 cm
HORIZONTAL SPEED
200 mmsec
PET DETECTOR
Solid state, 4032 avalanche photo diodes
DETECTOR SCINTILLATION MATERIAL
LSO, 28672 crystals
CRYSTAL SIZE
4 x 4 x 20 mm
DIMENSION H*W*D (gantry included)
335 x 230 x 242 cm (finshed covers)
COOLING SYSTEM
PET system: water; MRI system: water
Aautomatic, patient specific shim; active shim 3 linear and 5 non-linear channels (seond order)
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; Total system 110kW
| |  | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |