 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Multi Slab' found in 1 term [ ] and 19 definitions [ ] and 19 definitions [ ] ]
| previous 6 - 10 (of 20) nextResult Pages :  [1] [1]  [2 3 4] [2 3 4] |  | |  | Searchterm 'Multi Slab' was also found in the following service: | | | | |
|  |  |
| |
|
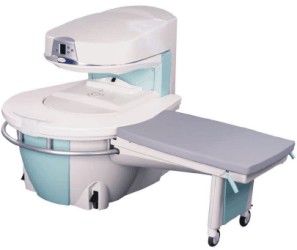
Manufactured by Esaote S.p.A.;
a low field open MRI scanner with permanent magnet for orthopedic use. The outstanding feature of this MRI system is a patient friendly design with 24 cm diameter, which allows the imaging of extremities and small body parts like shoulder MRI. The power consumption is around 1.3 kW and the needed minimum floor space is an area of 16 sq m.
At RSNA 2006 Hologic Inc. introduced a new dedicated extremity MRI scanner, the Opera. Manufactured by Esaote is the Opera a redesign of Esaote's 0.2 Tesla E-Scan XQ platform, which now enables complete imaging of all extremities, including hip and shoulder applications. 'Real-time positioning' reportedly speeds patient setup and reduces exam times.
Esaote North America and Hologic Inc are the U.S. distributors of this MRI device.
Device Information and Specification CLINICAL APPLICATION Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE IMAGING MODES Single, multislice, volume study, fast scan, multi slab2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm 4096 gray lvls, 256 lvls in 3D POWER REQUIREMENTS 2,0 kW; 110/220 V single phase | |  | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
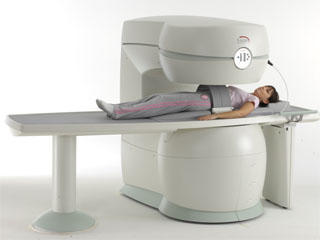
From Esaote S.p.A.;
Esaote introduced the S-SCAN at RSNA in November 2007. The S-SCAN is a dedicated joint and spine MR scanner derived from the company's earlier G-SCAN system. Unlike the G-SCAN, neither the patient table nor
the magnet can rotate from horizontal to vertical position. The patient table can only moved manually. Improved electronics, new coils for lumbar and cervical spine, new pulse sequences, a modified version of the magnet poles and gradient coils are used with a new software release in the S-SCAN.
Esaote North America is the exclusive U.S. distributor of this MRI device.
Device Information and Specification SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE POWER REQUIREMENTS 3 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'S-SCAN' (3).
| | |
• View the NEWS results for 'S-SCAN' (1).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
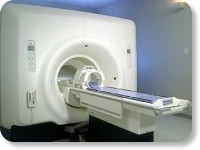
(Signa VH/i 3.0T)
With GE Healthcare
leading-edge technology in ultra-high-field imaging. The 3 T VH/i provides a platform for advanced applications in radiology, cardiology, psychology and psychiatry. Real-time image processing lets you acquire multislice whole brain images and map brain functions for research or surgical planning. And the 3 T Signa VH/i is flexible enough to provide clinicians with high performance they require. It can provide not only outstanding features in brain scanning and neuro-system research, but also a wide range of use in scanning breasts, extremities, the spine and the cardiovascular systems.
Device Information and Specification CLINICAL APPLICATION Whole body
T/R quadrature head, T/R quadrature body, T/R phased array extremity (opt) SE, IR, 2D/3D GRE, FGRE, RF-spoiled GRE, FSE, Angiography: 2D/3D TOF, 2D/3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer 100 Images/sec with Reflex100 MULTISLICE 100 Images/sec with Reflex100 2D 0.5-100mm in 0.1mm incremental 128x512 steps 32 phase encode H*W*D 260cm x 238cm x 265cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. Less than 0.14 L/hr liquid He | |  | |
• View the DATABASE results for 'Signa 3.0T™' (2).
| | | | |
|  |  | Searchterm 'Multi Slab' was also found in the following service: | | | | |
|  |  |
| |
|

From GE Healthcare;
GE's Signa Contour/i system uses the innovations like K4 technology and real-time interactive imaging.
This compact magnet with wide-flare gantry obtains high patient comfort with low costs.
Device Information and Specification CLINICAL APPLICATION Whole body Head and body coil standard; all other coils optional; open architecture makes system compatible with a wide selection of coils Standard: SE, IR, 2D/3D GRE and SPGR, Angiography;; 2D/3D TOF, 2D/3D Phase Contrast;; 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, optional: EPI, 2D/3D Fiesta, FGRET, Spiral2D 0.8 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 V STRENGTH SmartSpeed 23 mT/m, HiSpeed Plus 33 mT/m | |  | | | |
|  | |  |  |  |
| |
|

From GE Healthcare;
GE Healthcare has added the Signa HDe 1.5T™, a compact MRI device at an affordable price to its family of MRI products. It has a single electronic cabinet that can be positioned inside the scanner room rather than
in a separate equipment room. The Signa HDe 1.5T can be installed in the same physical location as 0.5T MRI systems with minimal construction costs. According to GE, the installation has been simplified to last only 7 days and has a 30 percent smaller footprint than a typical 1.5T system.
The 1.5T Signa™ HDe MRI system is substantially equivalent to the currently marketed GE 1.5T machines. The data acquisition system supports 1, 4, 8 independent receive channels and multiple independent coil elements per channel during a single acquisition series. The gradient specifications of HDe are lower than other GE Signa 1.5T MRI systems, but it can support clinical applications in cardiac and spectroscopy imaging.
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Compact short bore 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 less than 0.03 L/hr liquid helium | |  | |
• View the NEWS results for 'Signa HDe 1.5T™' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |