 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Musculoskeletal MRI' found in 0 term [ ] and 1 definition [ ] and 1 definition [ ], (+ 6 Boolean[ ], (+ 6 Boolean[ ] results ] results
| 1 - 5 (of 7) nextResult Pages :  [1] [1]  [2] [2] |  | |  | Searchterm 'Musculoskeletal MRI' was also found in the following services: | | | | |
|  |  |
| |
|
Knee and shoulder MRI exams are the most commonly requested musculoskeletal MRI scans. Other MR imaging of the extremities includes hips, ankles, elbows, and wrists. Orthopedic imaging requires very high spatial resolution for reliable small structure definition and therefore places extremely high demands on SNR.
Exact presentation of joint pathology expects robust and reliable fat suppression, often under difficult conditions like off-center FOV,
imaging at the edge of the field homogeneity or in regions with complex magnetic susceptibility.
MR examinations can evaluate meniscal dislocations, muscle fiber tears, tendon disruptions, tendinitis, and diagnose bone tumors and soft tissue masses. MR can also demonstrate acute fractures that are radiographically impossible to see. Evaluation of articular cartilage for traumatic injury or assessment of degenerative disease represents an imaging challenge, which can be overcome by high field MRI applications. Currently, fat-suppressed 3D spoiled gradient echo sequences and density weighted fast spin echo sequences are the gold-standard techniques used to assess articular cartilage.
Open MRI procedures allow the kinematic imaging of joints, which provides added value to any musculoskeletal MRI practice. This technique demonstrates the actual functional impingements or positional subluxations of joints. In knee MRI examinations, the kinematical patellar study can show patellofemoral joint abnormalities.
See also Open MRI, Knee MRI, Low Field MRI. | | | | | | | | | | | | | • Share the entry 'Imaging of the Extremities':    | | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
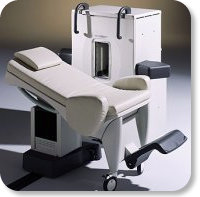
Developed by GE Lunar; the ARTOSCAN™-M is designed specifically for in-office musculoskeletal imaging. ARTOSCAN-M's compact, modular design allows placing within a clinical environment, bringing MRI to the patient. Patients remain outside the magnet at all times during the examinations, enabling constant patient-technologist contact. ARTOSCAN-M requires no special RF room, magnetic shielding, special power supply or air conditioning.
The C-SCAN™ (also known as Artoscan C) is developed from the ARTOSCAN™ - M, with a new computer platform.
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
SLICE THICKNESS
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240V
| |  | |
• View the DATABASE results for 'ARTOSCAN™ - M' (3).
| | | | |
|  | |  |  |  |
| |
|
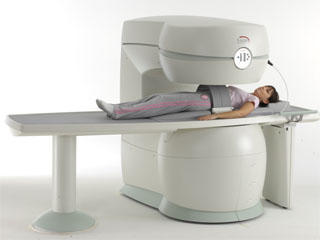
From Esaote S.p.A.;
Esaote introduced the S-SCAN at RSNA in November 2007. The S-SCAN is a dedicated joint and spine MR scanner derived from the company's earlier G-SCAN system. Unlike the G-SCAN, neither the patient table nor
the magnet can rotate from horizontal to vertical position. The patient table can only moved manually. Improved electronics, new coils for lumbar and cervical spine, new pulse sequences, a modified version of the magnet poles and gradient coils are used with a new software release in the S-SCAN.
Esaote North America is the exclusive U.S. distributor of this MRI device.
Device Information and Specification SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE POWER REQUIREMENTS 3 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'S-SCAN' (3).
| | |
• View the NEWS results for 'S-SCAN' (1).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Musculoskeletal MRI' was also found in the following services: | | | | |
|  |  |
| |
|
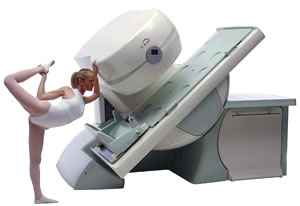
From Esaote S.p.A.;
Esaote introduced the new G-SCAN at the RSNA in Dec. 2004. The G-SCAN covers almost all musculoskeletal applications including the spine. The tilting gantry is designed for scanning in weight-bearing positions. This unique MRI scanner is developed in line with the Esaote philosophy of creating high quality MRI systems that are easy to install and that have a low breakeven point.
Device Information and Specification
SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
100 up to 350 mm, 25 mm displayed
POWER REQUIREMENTS
100/110/200/220/230/240 V
| |  | |
• View the DATABASE results for 'G-SCAN' (3).
| | | | |
|  | |  |  |  |
| |
|

The Esaote Group, founded in the 1980's, is a global leader in research, production, and marketing of medical diagnostic equipment and related services. Esaote offers integrated diagnostic solutions for ultrasound electromedical diagnostic systems, and MRI. The Esaote MR equipment is dedicated for specific clinical applications, as distinguished from conventional whole body MRI systems. This MRI product family is based on a unique, proprietary technology developed specifically for musculoskeletal imaging.
The Italian based company is a member of the Bracco Group.
Esaote North America is the exclusive U.S. distributor of MRI devices manufactured by Esaote.
MRI Scanners:
0.2T to 0.31T:
Vet-MR
(Based on the E Scan XQ, dedicated to the veterinarian practice.)
Contact Information
MAIL
ESAOTE S.p.A. MRI DIvision
Via Siffredi, 58
16153 Genova
Italy
| |  | |
• View the DATABASE results for 'Esaote S.p.A.' (5).
| | |
• View the NEWS results for 'Esaote S.p.A.' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |
|  | 1 - 5 (of 7) nextResult Pages :  [1] [1]  [2] [2] |
| |
|
| |
 | Look
Ups |
| |