 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | 'Phase Contrast Angiography' | |
Result : Searchterm 'Phase Contrast Angiography' found in 1 term [ ] and 8 definitions [ ] and 8 definitions [ ], (+ 19 Boolean[ ], (+ 19 Boolean[ ] results ] results
| previous 26 - 28 (of 28) Result Pages :  [1] [1]  [2] [2]  [3 4 5 6] [3 4 5 6] |  | | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: Head, body, cardiac, optional phased array: Spine, pediatric, 3rd party connector; Optional SENSE? coils for all applications
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: Spine, pediatric, 3rd party connector, Optional SENSE Coils: Flex-S-M-L, Flex Body, Flex Cardiac
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
TR
Min. 2.9 (Omni) msec, 1.6 (Power) msec
TE
Min. 1.0 (Omni) msec, 0.7 (Power) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm(Omni), 0.05 mm (Power)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
STRENGTH
23 mT/m (Omni), 30 (Power) mT/m
| |  | |
• View the DATABASE results for 'Intera 1.0T™' (2).
| | | | |
|  | |  |  |  |
| |
|
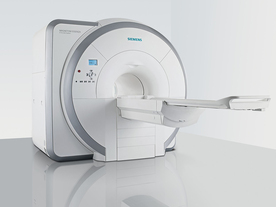
From Siemens Medical Systems;
Received FDA clearance in 2007.
The MAGNETOM Essenza is designed to combine high system performance with simple installation and power requirements to provide optimal operating costs for limited budgets. The standard system has up to 25 integrated coil elements and 8 independent radio frequency channels. Tim allows the combination of up to 4 different coils that reduce patient and coil repositioning.
The 1.5 Tesla system is designated for a complete range of clinical applications, including neurology, orthopedics, body imaging, angiography, cardiology, breast imaging, oncology and pediatric MRI.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Ultra-short bore
Head, spine, torso/ body coil, neurovascular, cardiac, neck, and multi-purpose flex coils. Peripheral vascular, breast, shoulder, knee, wrist, foot//ankle, TMJ optional.
CHANNELS (min. / max. configuration)
8, 16
MAGNET WEIGHT (gantry included)
4350 kg in operation
DIMENSION H*W*D (gantry included)
145 x 226 x 216 cm
COOLING SYSTEM
Water; single cryogen, 2 stage refrigeration
30 mT/m, 300 msec to 10 mT/m
Passive, active; first order standard
second order optional
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 45 kVA
| |  | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |