 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Phased Array Coil' found in 1 term [ ] and 8 definitions [ ] and 8 definitions [ ], (+ 15 Boolean[ ], (+ 15 Boolean[ ] results ] results
| previous 11 - 15 (of 24) nextResult Pages :  [1] [1]  [2] [2]  [3 4 5] [3 4 5] |  | |  | Searchterm 'Phased Array Coil' was also found in the following services: | | | | |
|  |  |
| |
|
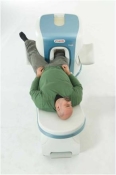
O-scan is manufactured and distributed by Esaote SpA
O-scan is a compact, dedicated extremity MRI system designed for easy installation and high throughput. The complete system fits in a 9' x 10' room, doesn't need for RF or magnetic shielding and it plugs in the wall. The 0.31T permanent magnet along with dual phased array RF coils, and advanced imaging protocols provide outstanding image quality and fast 25 minute complete examinations.
Esaote North America is the exclusive distributor of the O-scan system in the USA.
Device Information and Specification CLINICAL APPLICATION Dedicated Extremity
PULSE SEQUENCES
SE, HSE, HFE, GE, 2dGE, ME, IR, STIR, Stir T2, GESTIR, TSE, TME, FSE STIR, FSE ( T1, T2), X-Bone, Turbo 3DT1, 3D SHARC, 3D SST1, 3D SST2 2D: 2mm - 10 mm, 3D: 0.6 - 10 mm POWER REQUIREMENTS 100/110/200/220/230/240 | |  | | | |
|  | |  |  |  |
| |
|
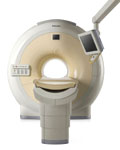
From Philips Medical Systems;
The clinical capabilities of MR will further expand. Inside and out, the Achieva is a friendly, open system designed for optimal patient comfort and maximized workflow with high functionality.
The Achieva 1.5T can be upgraded to Achieva I/T, with three configurations optimized for MR guided interventions and therapy:
•
Achieva I/T Neurosurgery
•
Achieva I/T Cardiovascular (or XMR - combining an Achieva 1.5T CV system and an X-Ray system)
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: Head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; optional phased array: Spine, pediatric, 3rd party connector; Optional SENSEâ„¢ coils for all applications
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera Achieva 1.5T™' (2).
| | | | |
|  | |  |  |  |
| |
|
(Signa VH/i 3.0T)
With GE Healthcare
leading-edge technology in ultra-high-field imaging. The 3 T VH/i provides a platform for advanced applications in radiology, cardiology, psychology and psychiatry. Real-time image processing lets you acquire multislice whole brain images and map brain functions for research or surgical planning. And the 3 T Signa VH/i is flexible enough to provide clinicians with high performance they require. It can provide not only outstanding features in brain scanning and neuro-system research, but also a wide range of use in scanning breasts, extremities, the spine and the cardiovascular systems.
Device Information and Specification CLINICAL APPLICATION Whole body
T/R quadrature head, T/R quadrature body, T/R phased array extremity (opt) SE, IR, 2D/3D GRE, FGRE, RF-spoiled GRE, FSE, Angiography: 2D/3D TOF, 2D/3D phase contrast vascular IMAGING MODES Single, multislice, volume study, fast scan, multi slab, cine, localizer 100 Images/sec with Reflex100 MULTISLICE 100 Images/sec with Reflex100 2D 0.5-100mm in 0.1mm incremental 128x512 steps 32 phase encode H*W*D 260cm x 238cm x 265cm POWER REQUIREMENTS 480 or 380/415, 3 phase ||
COOLING SYSTEM TYPE Closed-loop water-cooled grad. Less than 0.14 L/hr liquid He | |  | |
• View the DATABASE results for 'Signa 3.0T™' (2).
| | | | |
|  |  | Searchterm 'Phased Array Coil' was also found in the following services: | | | | |
|  |  |
| |
|
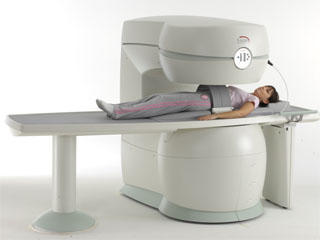
From Esaote S.p.A.;
Esaote introduced the S-SCAN at RSNA in November 2007. The S-SCAN is a dedicated joint and spine MR scanner derived from the company's earlier G-SCAN system. Unlike the G-SCAN, neither the patient table nor
the magnet can rotate from horizontal to vertical position. The patient table can only moved manually. Improved electronics, new coils for lumbar and cervical spine, new pulse sequences, a modified version of the magnet poles and gradient coils are used with a new software release in the S-SCAN.
Esaote North America is the exclusive U.S. distributor of this MRI device.
Device Information and Specification SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE POWER REQUIREMENTS 3 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'S-SCAN' (3).
| | |
• View the NEWS results for 'S-SCAN' (1).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
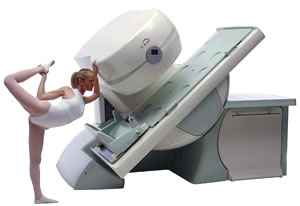
From Esaote S.p.A.;
Esaote introduced the new G-SCAN at the RSNA in Dec. 2004. The G-SCAN covers almost all musculoskeletal applications including the spine. The tilting gantry is designed for scanning in weight-bearing positions. This unique MRI scanner is developed in line with the Esaote philosophy of creating high quality MRI systems that are easy to install and that have a low breakeven point.
Device Information and Specification
SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
100 up to 350 mm, 25 mm displayed
POWER REQUIREMENTS
100/110/200/220/230/240 V
| |  | |
• View the DATABASE results for 'G-SCAN' (3).
| | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |