 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Shoulder MRI' found in 1 term [ ] and 2 definitions [ ] and 2 definitions [ ], (+ 18 Boolean[ ], (+ 18 Boolean[ ] results ] results
| previous 11 - 15 (of 21) nextResult Pages :  [1] [1]  [2 3 4 5] [2 3 4 5] |  | |  | Searchterm 'Shoulder MRI' was also found in the following services: | | | | |
|  |  |
| |
|
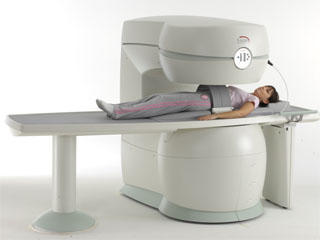
From Esaote S.p.A.;
Esaote introduced the S-SCAN at RSNA in November 2007. The S-SCAN is a dedicated joint and spine MR scanner derived from the company's earlier G-SCAN system. Unlike the G-SCAN, neither the patient table nor
the magnet can rotate from horizontal to vertical position. The patient table can only moved manually. Improved electronics, new coils for lumbar and cervical spine, new pulse sequences, a modified version of the magnet poles and gradient coils are used with a new software release in the S-SCAN.
Esaote North America is the exclusive U.S. distributor of this MRI device.
Device Information and Specification SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE POWER REQUIREMENTS 3 kW; 110/220 V single phase | |  | |
• View the NEWS results for 'S-SCAN' (1).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Shoulder MRI' was also found in the following service: | | | | |
|  |  |
| |
|
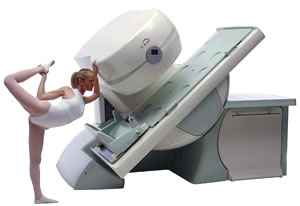
From Esaote S.p.A.;
Esaote introduced the new G-SCAN at the RSNA in Dec. 2004. The G-SCAN covers almost all musculoskeletal applications including the spine. The tilting gantry is designed for scanning in weight-bearing positions. This unique MRI scanner is developed in line with the Esaote philosophy of creating high quality MRI systems that are easy to install and that have a low breakeven point.
Device Information and Specification
SE, GE, IR, STIR, TSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
100 up to 350 mm, 25 mm displayed
POWER REQUIREMENTS
100/110/200/220/230/240 V
| |  | |
• View the DATABASE results for 'G-SCAN' (3).
| | | | |
|  | |  |  |  |
| |
|
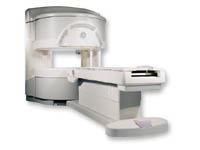
From GE Healthcare;
a friendly and less confining appearance targets the 7% of individuals who refuse to have an MRI because of claustrophobia. This open MRI system is also up to three times faster than other scanners, therefore the Signa OpenSpeed™ reducing exam time and scheduling
issues. In addition, a swing table provides better access and supports up to 500 pounds.
Device Information and Specification CLINICAL APPLICATION Whole body Standard: SE, IR, 2D/3D GRE and SPGR, Angiography: 2D/3D TOF, 2D/3D Phase Contrast;; 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, FGRET, Spiral, TensorTR 1.3 to 12000 msec in increments of 1 msec TE 0.4 to 2000 msec in increments of 1 msec 2D: 0.8mm - 20mm 3D: 0.1mm - 20mm 0.08 mm; 0.02 mm optional POWER REQUIREMENTS 200 - 480, 3-phase | |  | |
• View the DATABASE results for 'Signa OpenSpeed™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Shoulder MRI' was also found in the following services: | | | | |
|  |  |
| |
|

From GE Healthcare;
GE Healthcare has added the Signa HDe 1.5T™, a compact MRI device at an affordable price to its family of MRI products. It has a single electronic cabinet that can be positioned inside the scanner room rather than
in a separate equipment room. The Signa HDe 1.5T can be installed in the same physical location as 0.5T MRI systems with minimal construction costs. According to GE, the installation has been simplified to last only 7 days and has a 30 percent smaller footprint than a typical 1.5T system.
The 1.5T Signa™ HDe MRI system is substantially equivalent to the currently marketed GE 1.5T machines. The data acquisition system supports 1, 4, 8 independent receive channels and multiple independent coil elements per channel during a single acquisition series. The gradient specifications of HDe are lower than other GE Signa 1.5T MRI systems, but it can support clinical applications in cardiac and spectroscopy imaging.
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Compact short bore 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 less than 0.03 L/hr liquid helium | |  | |
• View the NEWS results for 'Signa HDe 1.5T™' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | Searchterm 'Shoulder MRI' was also found in the following service: | | | | |
|  |  |
| |
|
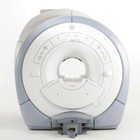
From GE Healthcare;
The GE Signa HDx MRI system is a whole body magnetic resonance scanner designed to support high resolution, high signal to noise ratio, and short scan times.
The 1.5T Signa HDx MR Systems is a modification of the currently marketed GE 1.5T machines, with the main difference being the change to the receive chain architecture that includes a thirty two independent receive channels, and allows for future expansion in 16 channel increments. The overall system has been improved with a simplified user interface
and a single 23" liquid crystal display, improved multi channel surface coil connectivity, and an improved image reconstruction architecture known as the Volume Recon Engine (VRE).
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Compact short bore Standard: SE, IR, 2D/3D GRE and SPGR, Angiography: 2D/3D TOF, 2D/3D Phase Contrast; 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, FGRET, Spiral, Tensor, 2D 0.7 mm to 20 mm; 3D 0.1 mm to 5 mm 128x512 steps 32 phase encode POWER REQUIREMENTS 480 or 380/415 less than 0.03 L/hr liquid helium | |  | | | |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |