 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  | Searchterm 'Spectroscopy' was also found in the following services: | | | | |
|  |  |
| |
|
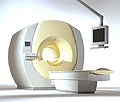
From Philips Medical Systems;
the Intera 3 T high field system, the first with a compact magnet, which is built on the same platform as the 1.5 T, is targeted to high-end neurological, orthopedic and cardiovascular imaging applications with maximum patient comfort and acceptance without compromising image quality and clinical performance. Useable for clinical routine and research.
The Intera systems offer diffusion tensor imaging ( DTI) fiber tracking that measures movement of water in the brain and can therefore detect areas of the brain where normal movement of water is disrupted.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; Optional phased array: spine;; Optional SENSE coils: Flex body, flex cardiac, neuro-vascular, head
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
TR
Min. 1.6 (Master) msec
TE
Min. 0.5 (Master) msec
RapidView Recon. greater than 500 @ 256 Matrix
0.1 mm (Omni), 0.05 mm (Power)
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
STRENGTH
30 (Master) mT/m
| |  | | | |
|  |  | Searchterm 'Spectroscopy' was also found in the following service: | | | | |
|  |  |
| |
|
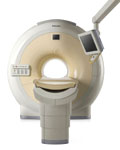
From Philips Medical Systems;
The clinical capabilities of MR will further expand. Inside and out, the Achieva is a friendly, open system designed for optimal patient comfort and maximized workflow with high functionality.
The Achieva 1.5T can be upgraded to Achieva I/T, with three configurations optimized for MR guided interventions and therapy:
•
Achieva I/T Neurosurgery
•
Achieva I/T Cardiovascular (or XMR - combining an Achieva 1.5T CV system and an X-Ray system)
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: Head, body, C1, C3; Optional: Small joint, flex-E, flex-R, endocavitary (L and S), dual TMJ, knee, neck, T/L spine, breast; optional phased array: Spine, pediatric, 3rd party connector; Optional SENSEâ„¢ coils for all applications
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera Achieva 1.5T™' (2).
| | | | |
|  | |  |  |  |
| |
|

From Philips Medical Systems;
Philips continues to expand the frontiers of utra high field MRI with the introduction of the new Intera Achieva 3.0T™. Its powerful future-safe platform shares all the advantages of the Achieva family and covers applications throughout the whole body.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
Passive and dynamic, 1st order std./2nd opt.
| |  | |
• View the DATABASE results for 'Intera Achieva 3.0T™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Spectroscopy' was also found in the following services: | | | | |
|  |  |
| |
|
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Short bore compact
Standard: Head, body, cardiac, optional phased array: Spine, pediatric, 3rd party connector; Optional SENSE? coils for all applications
SE, Modified-SE, IR (T1, T2, PD), STIR, FLAIR, SPIR, FFE, T1-FFE, T2-FFE, Balanced FFE, TFE, Balanced TFE, Dynamic, Keyhole, 3D, Multi Chunk 3D, Multi Stack 3D, K Space Shutter, MTC, TSE, Dual IR, DRIVE, EPI, Cine, 2DMSS, DAVE, Mixed Mode; Angiography: Inflow MRA, TONE, PCA, CE MRA
128 x 128, 256 x 256,512 x 512,1024 x 1024 (64 for Bold img)
Variable in 1% increments
Lum.: 120 cd/m2; contrast: 150:1
Variable (op. param. depend.)
POWER REQUIREMENTS
380/400 V
| |  | |
• View the DATABASE results for 'Intera Achieva CV™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Spectroscopy' was also found in the following service: | | | | |
|  |  |
| |
|
The owner of MRI equipment has to ensure that the equipment does fulfill the local requirements.
In some countries, the requirements are more stringent than in others; in other countries, they are nonexistent.
The user in general is unable to check power output, gradient strength, or even field strength.
The manufacturer has to cover authorized hardware and software updates after the initial installation and has to give guarantee for the requirements.
Specially designed computer programs usually supervise the power output of MRI devices and will not allow or will interrupt any imaging or spectroscopy procedure exceeding those limits considered safe.
See also European Medicines Agency, FDA information:
www.fda.gov/cdrh/safety/mrisafety.html | |  | |
• View the DATABASE results for 'Legal Requirements' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |