 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Tesla' found in 3 terms [ ] and 38 definitions [ ] and 38 definitions [ ] ]
| previous 31 - 35 (of 41) nextResult Pages :  [1] [1]  [2 3 4 5 6 7 8 9] [2 3 4 5 6 7 8 9] |  | |  | Searchterm 'Tesla' was also found in the following services: | | | | |
|  |  |
| |
|

If a device is to be labeled MR Safe, the following information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment (i.e. the MRI scan room) it does not pose an increased safety risk to the patient or other personnel,
•
a scientifically-based rationale for why data are not necessary to prove the safety of the device in the MR environment (for example, a passive device made entirely of a polymer known to be nonreactive in strong magnetic fields).
If a device is to be labeled MR Compatible, the following information should be provided:
•
Data demonstrating that when the device is introduced or used in the MRI environment, it is MR safe that it performs its intended function without performance degradation, and that it does not adversely affect the function of the MRI scanner (e.g. no significant image artifacts or noise). Any image artifact or noise due to the medical device should be quantified (e.g., % volume affected, signal to noise ratio),
•
a scientifically-based rationale for why data are not necessary to prove the compatibility of the device in the MRI environment.
Test Conditions:
The static magnetic field strength ( Gauss (G) or Tesla (T)) to which the device was tested and demonstrated to be MRI 'safe', 'compatible', or 'intended for use in' should be related to typical machine ratings (e.g. 0.5 T, 1.5 T, 2.0 T, and shielded or unshielded magnet, etc).
The same conditions should be used for the spatial gradient ( field strength per unit distance (i.e., G/cm)) in which the device was tested and demonstrated to be 'safe', 'compatible', or 'intended for use in'.
Also the RF transmitter power used during testing of the device, should be related to this typical machine ratings. | |  | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | |
• View the NEWS results for 'MR Compatibility' (2).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Tesla' was also found in the following services: | | | | |
|  |  |
| |
|
| |  | |
• View the DATABASE results for 'Magnetic Field Gradient' (28).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|
(B) Also called magnetic flux density with the SI unit tesla (T) usually denoted by the symbol B. The magnetic induction is the net magnetic effect from an externally applied magnetic field and the resulting magnetization.
The symbol H was used for the magnetic field (measured in amperes per meter (A/m)). However, this distinction is often ignored, and both quantities are often referred to as the magnetic field.
B is proportional to H (B = ÎĽH).
(ÎĽ is the magnetic permeability (in henries per meter) of the medium) | |  | |
• View the DATABASE results for 'Magnetic Induction' (5).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  |  | Searchterm 'Tesla' was also found in the following services: | | | | |
|  |  |
| |
|

Founded in June 1997, ONI was a privately held company headquartered in Wilmington, Massachusetts. ONI was focused on addressing the new healthcare economics by delivering the next generation of MRI machines: high field, low cost dedicated purpose systems. The company's first offering was the OrthOne™. Further developments lead to the MSK Extreme™, a 1.0 Tesla dedicated purpose MR system with v-SPEC™ Technology and DICOM Conformance for extremity applications.
In October 2009 GE Healthcare bought ONI Medical Systems for US$ 17 Billion.
MRI Scanners:
| |  | |
• View the DATABASE results for 'ONI Medical Systems, Inc.' (2).
| | |
• View the NEWS results for 'ONI Medical Systems, Inc.' (4).
| | | | |
|  |  | Searchterm 'Tesla' was also found in the following services: | | | | |
|  |  |
| |
|
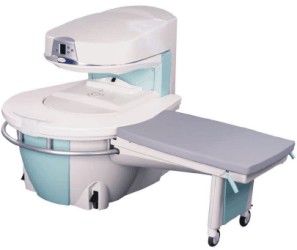
Manufactured by Esaote S.p.A.;
a low field open MRI scanner with permanent magnet for orthopedic use. The outstanding feature of this MRI system is a patient friendly design with 24 cm diameter, which allows the imaging of extremities and small body parts like shoulder MRI. The power consumption is around 1.3 kW and the needed minimum floor space is an area of 16 sq m.
At RSNA 2006 Hologic Inc. introduced a new dedicated extremity MRI scanner, the Opera. Manufactured by Esaote is the Opera a redesign of Esaote's 0.2 Tesla E-Scan XQ platform, which now enables complete imaging of all extremities, including hip and shoulder applications. 'Real-time positioning' reportedly speeds patient setup and reduces exam times.
Esaote North America and Hologic Inc are the U.S. distributors of this MRI device.
Device Information and Specification CLINICAL APPLICATION Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE IMAGING MODES Single, multislice, volume study, fast scan, multi slab2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm 4096 gray lvls, 256 lvls in 3D POWER REQUIREMENTS 2,0 kW; 110/220 V single phase | |  | |
• View the DATABASE results for 'Opera (E-SCAN™ XQ)' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |