 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'contact' found in 0 term [ ] and 42 definitions [ ] and 42 definitions [ ] ]
| previous 11 - 15 (of 42) nextResult Pages :  [1 2 3 4 5 6 7 8 9] [1 2 3 4 5 6 7 8 9] |  | |  | Searchterm 'contact' was also found in the following services: | | | | |
|  |  |
| |
|

It is important to remember when working around a superconducting magnet that the magnetic field is always on. Under usual working conditions the field is never turned off. Attention must be paid to keep all ferromagnetic items at an adequate distance from the magnet. Ferromagnetic objects which came accidentally under the influence of these strong magnets can injure or kill individuals in or nearby the magnet, or can seriously damage every hardware, the magnet itself, the cooling system, etc..
See MRI resources Accidents.
The doors leading to a magnet room should be closed at all times except when entering or exiting the room. Every person working in or entering the magnet room or adjacent rooms with a magnetic field has to be instructed about the dangers. This should include the patient, intensive-care staff, and maintenance-, service- and cleaning personnel, etc..
The 5 Gauss limit defines the 'safe' level of static magnetic field exposure. The value of the absorbed dose is fixed by the authorities to avoid heating of the patient's tissue and is defined by the specific absorption rate.
Leads or wires that are used in the magnet bore during imaging procedures, should not form large-radius wire loops. Leg-to-leg and leg-to-arm skin contact should be prevented in order to avoid the risk of burning due to the generation of high current loops if the legs or arms are allowed to touch. The patient's skin should not be in contact with the inner bore of the magnet.
The outflow from cryogens like liquid helium is improbable during normal operation and not a real danger for patients.
The safety of MRI contrast agents is tested in drug trials and they have a high compatibility with very few side effects. The variations of the side effects and possible contraindications are similar to X-ray contrast medium, but very rare. In general, an adverse reaction increases with the quantity of the MRI contrast medium and also with the osmolarity of the compound.
See also 5 Gauss Fringe Field, 5 Gauss Line, Cardiac Risks, Cardiac Stent, dB/dt, Legal Requirements, Low Field MRI, Magnetohydrodynamic Effect, MR Compatibility, MR Guided Interventions, Claustrophobia, MRI Risks and Shielding. | | | | | | | | | • For this and other aspects of MRI safety see our InfoSheet about MRI Safety. | | | • Patient-related information is collected in our MRI Patient Information.
| | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  |  | MRI Safety Resources | | | | |
|  |  |  |
| |
|

Philips Medical System is the diagnostics business of Royal Philips Electronics of the Netherlands, one of the world's biggest electronics companies and Europe's largest. Philips is quoted on the NYSE (symbol: PHG), London, Frankfurt, Amsterdam and other stock exchanges.
On October 19, 2001, Philips Medical Systems completed a 3-year acquisition strategy through its purchase of Marconi Medical Systems. Marconi Medical Systems offered leading multislice CT, MRI, and Nuclear Gamma Camera systems to medical institutions around the world. As well as new 3.0T developments, Philips is also in collaboration with researchers at the University of Nottingham, with the intention of developing an ultrahigh field strength clinical 7.0T whole body MR system.
MRI Scanners:
0.23T to 1.0T:
1.5T:
3.0T to 7.0T:
Hybrid Scanners:
Contact Information MAIL Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810-1099
USA | |  | |
• View the DATABASE results for 'Philips Medical Systems' (14).
| | |
• View the NEWS results for 'Philips Medical Systems' (12).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
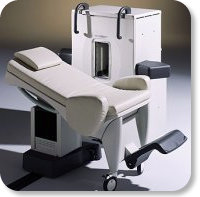
Developed by GE Lunar; the ARTOSCAN™-M is designed specifically for in-office musculoskeletal imaging. ARTOSCAN-M's compact, modular design allows placing within a clinical environment, bringing MRI to the patient. Patients remain outside the magnet at all times during the examinations, enabling constant patient-technologist contact. ARTOSCAN-M requires no special RF room, magnetic shielding, special power supply or air conditioning.
The C-SCAN™ (also known as Artoscan C) is developed from the ARTOSCAN™ - M, with a new computer platform.
Device Information and Specification
CLINICAL APPLICATION
Dedicated extremity
SE, GE, IR, STIR, FSE, 3D CE, GE-STIR, 3D GE, ME, TME, HSE
SLICE THICKNESS
2D: 2 mm - 10 mm;
3D: 0.6 mm - 10 mm
4,096 gray lvls, 256 lvls in 3D
POWER REQUIREMENTS
100/110/200/220/230/240V
| |  | |
• View the DATABASE results for 'ARTOSCAN™ - M' (3).
| | | | |
|  |  | Searchterm 'contact' was also found in the following services: | | | | |
|  |  |
| |
|

California-based research and development company. Alliance has several patents regarding ' microbubble' compositions such as Imagent® that are intended to enhance ultrasound imaging and Imagent GI as a gastrointestinal MRI contrast agent. Another product is Oxygent™, a synthetic 'blood substitute'.
MRI Contrast Agents:
Contact Information
MAIL
Alliance Pharmaceutical Corp.
6175 Lusk Blvd.
San Diego, CA 92121
USA
| |  | |
• View the DATABASE results for 'Alliance Pharmaceutical Corp.' (2).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|

Aurora Imaging Technology, Inc., a private company, is located in North Andover, Massachusetts. As a leading innovator in the breast imaging industry, the company provides with its Aurora System the detection, diagnosis, biopsy and treatment of breast cancer. Aurora Imaging Technology Inc. develops and manufactures the Aurora® 1.5T Dedicated Breast MR System, the first and only FDA approved MRI system designed specifically for breast imaging.
MRI Scanners:
Contact Information
MAIL
Aurora Imaging Technology Inc
39 High Street
North Andover, MA 01845
USA
| |  | |
• View the DATABASE results for 'Aurora Imaging Technology, Inc.' (2).
| | |
• View the NEWS results for 'Aurora Imaging Technology, Inc.' (5).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |
|  | | |
|
| |
 | Look
Ups |
| |