 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | | |  |
Result : Searchterm 'mtc' found in 0 term [ ] and 28 definitions [ ] and 28 definitions [ ] ]
| 1 - 5 (of 28) nextResult Pages :  [1 2 3 4 5 6] [1 2 3 4 5 6] |  | |  | Searchterm 'mtc' was also found in the following service: | | | | |
|  |  |
| |
|
( MTC) This MRI method increases the contrast by removing a portion of the total signal in tissue. An off resonance radio frequency (RF) pulse saturates macromolecular protons to make them invisible (caused by their ultra-short T2* relaxation times). The MRI signal from semi-solid tissue like brain parenchyma is reduced, and the signal from a more fluid component like blood is retained.
E.g., saturation of broad spectral lines may produce decreases in intensity of lines not directly saturated, through exchange of magnetization between the corresponding states; more closely coupled states will show a greater resulting intensity change.
Magnetization transfer techniques make demyelinated brain or spine lesions (as seen e.g. in multiple sclerosis) better visible on T2 weighted images as well as on gadolinium contrast enhanced T1 weighted images.
Off resonance makes use of a selection gradient during an off resonance MTC pulse. The gradient has a negative offset frequency on the arterial side of the imaging volume (caudally more off resonant and cranially less off resonant). The net effect of this type of pulse is that the arterial blood outside the imaging volume will retain more of its longitudinal magnetization, with more vascular signal when it enters the imaging volume. Off resonance MTC saturates the venous blood, leaving the arterial blood untouched.
On resonance has no effect on the free water pool but will saturate the bound water pool and is the difference in T2 between the pools. Special binomial pulses are transmitted causing the magnetization of the free protons to remain unchanged. The z-magnetization returns to its original value. The spins of the bound pool with a short T2 experience decay, resulting in a destroyed magnetization after the on resonance pulse.
See also Magnetization Transfer. | |  | | | | • Share the entry 'Magnetization Transfer Contrast':    | | | | | | | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|

From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0.3 T field-strength magnet and phased array coils deliver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  | |  |  |  |
| |
|
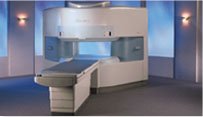
From Hitachi Medical Systems America, Inc.;
the AIRIS made its debut in 1995. Hitachi followed up with the AIRIS II system, which has proven equally successfully. 'All told, Hitachi has installed more than 1,000 MRI systems in the U.S., holding more than 17 percent of the total U.S. MRI installed base, and more than half of the installed base of open MR systems,' says Antonio Garcia, Frost and Sullivan industry research analyst.
Now Altaire employs a blend of innovative Hitachi features called VOSI™ technology, optimizing each sub-system's performance in concert with the
other sub-systems, to give the seamless mix of high-field performance
and the patient comfort, especially for claustrophobic patients, of open MR systems.
Device Information and Specification
CLINICAL APPLICATION
Whole body
DualQuad T/R Body Coil, MA Head, MA C-Spine, MA Shoulder, MA Wrist, MA CTL Spine, MA Knee, MA TMJ, MA Flex Body (3 sizes), Neck, small and large Extremity, PVA (WIP), Breast (WIP), Neurovascular (WIP), Cardiac (WIP) and MA Foot//Ankle (WIP)
SE, GE, GR, IR, FIR, STIR, ss-FSE, FSE, DE-FSE/FIR, FLAIR, ss/ms-EPI, ss/ms EPI- DWI, SSP, MTC, SE/GE-EPI, MRCP, SARGE, RSSG, TRSG, BASG, Angiography: CE, PC, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 3.6 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 8 - 250msec IR: 5.2 -7,680msec GE: 1.8 - 2,000 msec FSE: 5.2 - 7,680
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
COOLING SYSTEM TYPE
Water-cooled
3.1 m lateral, 3.6 m vertical
| |  | |
• View the DATABASE results for 'Altaire™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'mtc' was also found in the following service: | | | | |
|  |  |
| |
|
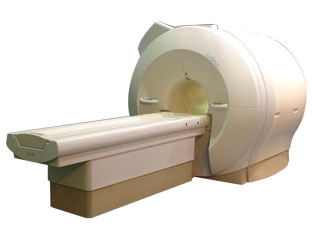
'Next generation MRI system 1.5T CHORUS developed by ISOL Technology is optimized for both clinical diagnostic imaging and for research development.
CHORUS offers the complete range of feature oriented advanced imaging techniques- for both clinical routine and research. The compact short bore magnet, the patient friendly design and the gradient technology make the innovation to new degree of perfection in magnetic resonance.'
Device Information and Specification
CLINICAL APPLICATION
Whole body
Spin Echo, Gradient Echo, Fast Spin Echo,
Inversion Recovery ( STIR, Fluid Attenuated Inversion Recovery), FLASH, FISP, PSIF, Turbo Flash ( MPRAGE ),TOF MR Angiography, Standard echo planar imaging package (SE-EPI, GE-EPI), Optional:
Advanced P.A. Imaging Package (up to 4 ch.), Advanced echo planar imaging package,
Single Shot and Diffusion Weighted EPI, IR/FLAIR EPI
STRENGTH
20 mT/m (Upto 27 mT/m)
| |  | |
• View the DATABASE results for 'CHORUS 1.5T™' (2).
| | | | |
|  | |  |  |  |
| |
|
From Philips Medical Systems;
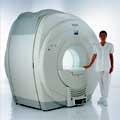
Philips Infinion 1.5 T is designed to maximize the efficiency and quality of patient care. Developed with the patient in mind, the Infinion is the shortest and most open 1.5T scanner available. The unique 'ultra short' 1.4 m magnet assures patient comfort and acceptance without compromising image quality and clinical performance.
Device Information and Specification
CLINICAL APPLICATION
Whole body
CONFIGURATION
Ultra short bore
Head, head / neck, integrated C-spine, L/T spine array, small large GP coils, body flex array, torso pelvis array, breast array, endocavitary, shoulder array, lower extremity, hand / wrist, cardiac, PV array
SE, TSE, SS TSE, EPI, IR, STIR, FLAIR, FFE, TFE, T1 TFE, T2 TFE, Presat, Fatsat, MTC, Diff-opt., Angiography: PCA, MCA, TOF
IMAGING MODES
Single slice, single volume, multi slice, multi volume
80 images/sec std.; up to320 opt.@256
H*W*D
233 (lead fitted) x 198 x 140 cm
POWER REQUIREMENTS
400/480 V
COOLING SYSTEM TYPE
Closed loop, chilled water
| |  | |
• View the DATABASE results for 'Infinion 1.5T™' (2).
| | | | |
|  | |  |  |
|  | |
|  | 1 - 5 (of 28) nextResult Pages :  [1 2 3 4 5 6] [1 2 3 4 5 6] |
| |
|
| |
 | Look
Ups |
| |