 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | 'Siemens Medical Systems' | |
Result : Searchterm 'Siemens Medical Systems' found in 1 term [ ] and 21 definitions [ ] and 21 definitions [ ] ]
| 1 - 5 (of 22) nextResult Pages :  [1] [1]  [2 3 4 5] [2 3 4 5] |  | | |  |  |  |
| |
|

The range of diagnostics and imaging systems of Siemens Medical Systems covers ultrasound, nuclear medicine, angiography, magnetic resonance, computer tomography and patient monitoring. Siemens is one of the three leading MRI manufacturers, which together account for approximately 80 percent of the MRI machines installed worldwide. Siemens currently offers the Allegra 3T MRI, which is for head scanning only, but the company will also be launching the Trio MRI, a 3T whole body scanner.
Siemens has formed partnerships with more than ten research institutions and private practitioners to define a comprehensive MRI examination and compare MR to currently established cardiovascular modalities, thereby defining optimal diagnosis and treatment.
MRI Scanners:
0.2T to 1.0T:
1.5T:
3.0T to 7.0T:
Hybrid Scanners:
Mobile Solutions:
•
MAGNETOM Espree 1.5T, MAGNETOM Avanto 1.5T and MAGNETOM ESSENZA 1.5T are also offered by Siemens on certified trailers.
Contact Information MAIL
Siemens Medical Solutions
Health Services Corporation
51 Valley Stream Parkway
Malvern, PA 19355
USA | |  | | | | • Share the entry 'Siemens Medical Systems':    | | |
• View the NEWS results for 'Siemens Medical Systems' (3).
| | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
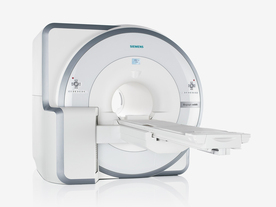
FDA cleared and CE Mark 2011.
The Biograph mMR has a fully-integrated design for simultaneous PET/MRI imaging. The dedicated hardware includes solid-state, avalanche photodiode PET detector and adapted, PET-compatible MR coils.
The possibility of truly simultaneous operation allows the acquisition of several magnetic resonance imaging ( MRI) sequences during the positron emission tomography (PET) scan, without increasing the examination time.
See also Hybrid Imaging.
Device Information and Specification
CLINICAL APPLICATION
Whole Body
CONFIGURATION
Simultaneous PET/MRI
26 cm (typical overlap 23%)
A-P 45, R-L 50, H-F 50 cm
PET RING DIAMETER
65.6 cm
PATIENT SCAN RANGE
199 cm
HORIZONTAL SPEED
200 mmsec
PET DETECTOR
Solid state, 4032 avalanche photo diodes
DETECTOR SCINTILLATION MATERIAL
LSO, 28672 crystals
CRYSTAL SIZE
4 x 4 x 20 mm
DIMENSION H*W*D (gantry included)
335 x 230 x 242 cm (finshed covers)
COOLING SYSTEM
PET system: water; MRI system: water
Aautomatic, patient specific shim; active shim 3 linear and 5 non-linear channels (seond order)
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; Total system 110kW
| |  | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
 [This entry is marked for removal.]
[This entry is marked for removal.]
(July 20, 2009 - EPIX Pharmaceuticals, Inc. announced today that, in light of the company's lack of capital and inability to obtain additional financing or consummate a strategic transaction, it has entered into an Assignment for the Benefit of Creditors, effective immediately, in accordance with Massachusetts law).
EPIX has been a specialty pharmaceutical firm developing targeted contrast agents to improve the capability of MRI as a diagnostic tool for a variety of diseases. Gadofosveset trisodium (formerly MS-325, Vasovist™, now ABLAVARt™), is an injectable intravascular contrast agents designed for multiple vascular imaging applications, including peripheral vascular disease and coronary artery disease.
EPIX conducted a pivotal Phase III trial for the detection of peripheral vascular disease, as well as a Phase II feasibility trial for coronary artery disease diagnosis.
To ensure rapid development and adoption of gadofosveset trisodium into clinical practice upon regulatory approval, EPIX pursued an aggressive product development plan and commercialization strategy. The Company established an exclusive, worldwide sales and marketing agreement with Bayer Schering Pharma AG. EPIX also established corporate collaborations with GE Healthcare, Philips Medical Systems and Siemens Medical Systems, the three leading MRI manufacturers, which together account for approximately 80 percent of the MRI machines installed worldwide.
EPIX had other MRI contrast agents under development, most significantly a novel prototype blood clot agent ( EP-2104R). Potential clinical applications for this type of agent include detection of deep venous thrombosis, pulmonary embolism and blood clots in the coronary and carotid arteries. Currently, there is no high resolution imaging technique to directly visualize blood clots in patients with suspected cardiovascular disease.
| |  | |
• View the DATABASE results for 'EPIX Pharmaceuticals, Inc.' (7).
| | |
• View the NEWS results for 'EPIX Pharmaceuticals, Inc.' (69).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |  |
| |
|
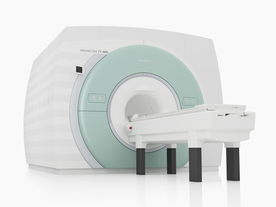
From Siemens Medical Systems;
The MAGNETOM 7T is designed as an open research platform. 7T MRI provides anatomical detail at the submillimiter scale, enhanced contrast mechanisms, outstanding spectroscopy performance, ultra-high resolution functional imaging ( fMRI), multinuclear whole-body MRI and functional information.
This ultra high field (UHF) MRI device is a research system and not cleared, approved or licensed in any jurisdiction for patient examinations.
Device Information and Specification
CLINICAL APPLICATION
Whole body
High-performance, ultra high field coils available. Integration and support for coil developments.
CHANNELS (min. / max. configuration)
32, optional 8 channels TX array
40 x 40 x 30 cm³ less than 8% nonlinearity
MAGNET WEIGHT (gantry included)
35017 kg
DIMENSION H*W*D (gantry included)
320 x 240 x 317,5 cm
MAX. AMPLITUDE
up to 70 mT/m
Up to 3rd order shim coils, user configurable B0 shim ? B0 maps and ROI definition
POWER REQUIREMENTS
2000 Volts, 650A
| |  | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
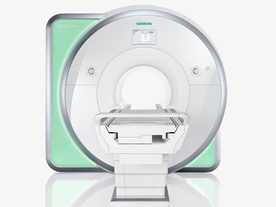
From Siemens Medical Systems;
Received FDA clearance in 2010.
The MAGNETOM Aera is a patient friendly, comfortable 1.5 Tesla MRI system with advanced radio frequency chain.
The system is equipped with the Tim 4G and Dot system (Total imaging matrix + Day optimizing throughput), to enhance both productivity and image quality.
Tim 4G technology provides improved SNR. The standard system configuration of 48 radio frequency channels and 204 coil elements creates an imaging matrix that allows maximum use of coil elements at full field of view. Dot provides improved image consistency through new features like auto align, auto FoV and automatic bolus detection.
Device Information and Specification
CLINICAL APPLICATION
Whole body
Head, spine, torso/ body coil, neurovascular, cardiac, neck, shoulder, knee, wrist, foot//ankle and multi-purpose flex coils. Peripheral vascular, breast, shoulder. Up to 60% more SNR with Tim 4G.
CHANNELS (min. / max. configuration)
48, 64
MINIMUM TE
3-D GRE: 0.22 (256 matrix), Ultra-short TE
At isocenter: L-R 70 cm, A-P (with table) 55 cm
MAGNET WEIGHT (gantry included)
3121 kg
DIMENSION H*W*D (gantry included)
145 x 231 x 219 cm
MAX. AMPLITUDE
33 or 45 mT/m
3 linear with 20 coils, 5 nonlinear 2nd-order
POWER REQUIREMENTS
380 / 400 / 420 / 440 / 460 / 480 V, 3-phase + ground; 85 kVA
| |  | | | |
|  | |  |  |
|  | 1 - 5 (of 22) nextResult Pages :  [1] [1]  [2 3 4 5] [2 3 4 5] |
| |
|
| |
 | Look
Ups |
| |