 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'tof' found in 0 term [ ] and 40 definitions [ ] and 40 definitions [ ] ]
| previous 36 - 40 (of 40) Result Pages :  [1 2 3 4 5 6 7 8] [1 2 3 4 5 6 7 8] |  | |  | Searchterm 'tof' was also found in the following services: | | | | |
|  |  |
| |
|
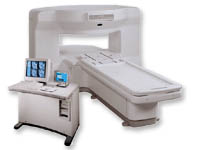
From GE Healthcare;
the New Signa Profile/i is a patient friendly open MRI system that virtually eliminates patient anxiety and claustrophobia, without compromising diagnostic utility.
Device Information and Specification CLINICAL APPLICATION Whole body Integrated transmit body coil, body flex sizes: M, L, XL, quadrature, head coil quadrature, 4 channel neurovascular array, 8 channel CTL array, quad. c- spine, 2 channel shoulder array, extremity coil, 3 channel wrist array, 4 channel breast array, 6, 9, 11 inch general purpose loop coils Standard: SE, IR, 2D/3D GRE and SPGR, Angiography: 2D/3D TOF, 2D/3D phase contrast; 2D/3D FSE, 2D/3D FRFSE, FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, fat/water separation, T1 FLAIRIMAGING MODES Localizer, single slice, multislice, volume, fast, POMP, multi slab, cine, slice and frequency zip, extended dynamic range, tailored RF TR 6 to 12000 msec in increments of 1 msec TE 1.3 to 2000 msec in increments of 1 msec 2D: 2.7mm - 20mm 3D: 0.2mm - 5mm 0.08 mm; 0.02 mm optional 10,000 kg w/gradient enclosure POWER REQUIREMENTS 200 - 480, 3-phase COOLING SYSTEM TYPE None required | |  | | | |
|  | |  |  |  |
| |
|

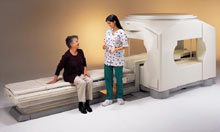
From GE Healthcare;
The Signa SP 0.5T™ is an open MRI magnet that is designed for use in interventional radiology and intra-operative imaging. The vertical gap configuration increases patient positioning options, improves patient observation, and allows continuous access to the patient during imaging.
The magnet enclosure also incorporates an intercom, patient observation video camera, laser patient alignment lights, and task lighting in the imaging volume.
Device Information and Specification CLINICAL APPLICATION Whole body Integrated transmit and receive body coil; optional rotational body coil, head; other coils optional; open architecture makes system compatible with a wide selection of coilsarray Standard: SE, IR, 2D/3D GRE and SPGR, 2D/3D TOF, 2D/3D FSE, 2D/3D FGRE and FSPGR, SSFP, FLAIR, EPI, optional: 2D/3D Fiesta, true chem sat, fat/water separation, single shot diffusion EPI IMAGING MODES Localizer, single slice, multislice, volume, fast, POMP, multi slab, cine, slice and frequency zip, extended dynamic range, tailored RF TR 1.3 to 12000 msec in increments of 1 msec TE 0.4 to 2000 msec in increments of 1 msec 2D: 1.4mm - 20mm 3D: 0.2mm - 20mm POWER REQUIREMENTS 200 - 480, 3-phase | |  | |
• View the DATABASE results for 'Signa SP 0.5T™ Open Configuration' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
From Toshiba America Medical Systems Inc.;
the Ultra™ system was developed to help healthcare providers be more competitive by delivering greater patient comfort and a broad range of clinical capabilities, says Anita Bowler, product manager, MRI Business Unit, Toshiba America Medical Systems Inc With its unique, powerful gradient technology, the Ultra™ performs advanced clinical studies and consistently provides high-resolution images that are typically associated with high field MRI systems. At the same time, the Ultra™ offers a truly open feeling that makes patients more relaxed, especially those with claustrophobic tendencies.
Device Information and Specification CLINICAL APPLICATION Whole Body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI, Single shot EPI diffusion, True SSFP, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC, Black Blood MRA POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Cryogenless | |  | | | |
|  |  | Searchterm 'tof' was also found in the following services: | | | | |
|  |  |
| |
|
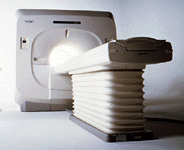
From Toshiba America Medical Systems Inc.;
VISART™ series is a 1.5 Tesla superconducting MRI system that has been designed to meet the expanding role of MRI in today's clinical environment. The system utilizes innovative technologies such as digital RF, high speed actively shielded gradients and optimized RF coils, which support a wide range of MRI developments. The Visart, an early type of Toshiba Medical Systems Inc., can be retrofitted or upgraded to the Excelart configuration.
Device Information and Specification CLINICAL APPLICATION Whole body Quadrature, solenoid and multi-channel configurations SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, Hybrid EPI, Multi Shot EPI; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Closed-loop water-cooled | |  | |
• View the DATABASE results for 'VISART™' (2).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|
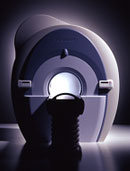
From Toshiba America Medical Systems Inc.;
With its high-field strength, the Vantage™ delivers the clinical capabilities and image quality expected by cardiologists, while simultaneously offering patients a more comfortable and non-invasive option, said Dane Peshe, director, MRI Business Unit, Toshiba America Medical Systems. Vantage™ supports a full complement of cardiovascular imaging studies, ranging from stroke evaluation to peripheral vascular imaging. Additionally, the ultra short bore design offers patients a greater feeling of openness that reduces claustrophobic sensations, while Toshiba's exclusive, patented Pianissimo™ technology reduces scan noise by as much as 90 percent for a more pleasant experience.'
Device Information and Specification CLINICAL APPLICATION Whole body CONFIGURATION Ultra short bore SE, FE, IR, FastSE, FastIR, FastFLAIR, Fast STIR, FastFE, FASE, EPI, SuperFASE; Angiography: 2D(gate/non-gate)/3D TOF, SORS-STC IMAGING MODES Single, multislice, volume study 32-1024, phase;; 64-1024, freq. POWER REQUIREMENTS 380/400/415/440/480 V COOLING SYSTEM TYPE Closed-loop water-cooled Liquid helium: approx. less than 0.05 L/hr Passive, active, auto-active | |  | |
• View the DATABASE results for 'Vantage™' (2).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
| |
|  | |  |  |
|  | |
|  | | |
|
| |
 | Look
Ups |
| |